10. Procedure
10.1 To a 250-mL Erlenmeyer flask add 50 +/- 0.1 g of engine coolant and approximately 30 mL of water. Make the solution strongly alkaline by the addition of 5 mL of 20 mass % aqueous NaOH solution. Add 5 mL of 30 mass % aqueous H2O2 with stirring, then connect the flask to a reflux condenser and reflux for 30 min. Cool the solution to room temperature and transfer the contents quantitatively to a 200-mL volumetric flask and dilute to volume with water.
10.2 In a 200-mL volumetric flask, prepare a blank by adding 5 mL of 30 mass % aqueous H2O2 and the same amount of 20 mass % aqueous NaOH solution used in 10.1 and dilute to 200 mL with water.
10.3 Pipet 100 mL ofthe glacial acetic acid titration solvent into each of two beakers. Accurately pipet 20 mL of the treated engine coolant sample into one beaker and 20 mL of the blank (10.2) into the second beaker and allow to dissolve. Titrate the contents of each beaker potentiometrically with 0.01 N AgNO3 solution while stirring slowly with a magnetic stirrer. The equilibration of electrode potentials is slow in the vicinity of the end point; therefore, the titrant should be added in 0.02-mL increments in this region and sufficient time allowed for stable readings to be obtained.
11. Calculation
11.1 Chloride Content:
11.1.1 Prepare a titration curve by plotting potential readings, using 2 mV per division, against the corresponding volume of titrant added, using 0.02 mL per division. The end point is selected at the middle of the steepest portion of the curve (see Fig. 1).
11.1.2 Calculate ppm of the chloride ion in the original engine coolant sample as follows:
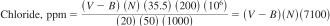
where:
V = titrant used, mL, in the engine coolant sample,
B = titrant used, mL, in the blank sample, and
N = normality of titrant.

