Introduction
There is not one single corrosive sulfur compound that is responsible for all corrosive sulfur issues that are present in all mineral oil filled electrical apparatus. Depending on the oil, there can be tens to hundreds of different sulfur compounds present in the oil. Of these, only a small fraction are corrosive or are compounds that can degrade from stable species into ones that are reactive. This is usually based on time and temperature. Only a very few corrosive sulfur compounds have been identified of which dibenzyl disulfide (DBDS) is one. This paper only concentrates on DBDS. The reason is that it has been found in many oils that have resulted in recent failures (2000-2007) of transformers or reactors due to corrosive sulfur attack and the formation of copper sulfide.
There are five main classes of sulfur compounds found in crude oil, but not all types are considered to be corrosive or reactive (see Table 1). Elemental sulfur and sulfur compounds in concentrations up to 20 percent are present in the crude oil used to make transformer oil.
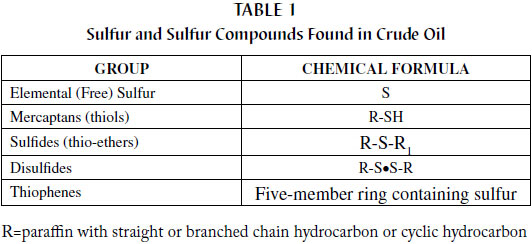
Sulfur is commonly found in crude oil, as it is a common element in the earth's crust. As shown in Table 1, elemental sulfur and the sulfur-containing mercaptans are very reactive followed by sulfides. Reactive sulfur is mainly in the form of organic sulfur compounds like R-SH, where the sulfur is attached at the end of an organic molecule. When the molecule is more complex, for instance when the sulfur is surrounded or contained within the molecule then the sulfur compounds are more stable and less reactive, like in R-S•S-R. Just a few years ago, disulfides (of which DBDS belongs) were once thought to be very stable but it has been found that the disulfide linkage can be susceptible to cleavage resulting in the production of mercaptans. Thiophenes are the most stable of all these sulfur compounds. Research at Doble has shown that even thiophenes will break down given enough time and thermal stress. Whether they form corrosive sulfur compounds or not is unknown. It is known that a large percentage of the breakdown products will reform into smaller thiophene compounds. Crude selection and the refining process are the two main factors that dictate the presence of any of the five sulfur groups in a finished transformer oil.
Presently, the refining techniques are such that detectable amounts of elemental sulfur and mercaptans are very rare in a newly finished transformer oil. Other certain types of sulfur containing products are considered advantageous as they may provide some degree of oxidation stability.
DBDS in itself might or might not be corrosive. Some researchers suggest that a DBDS-copper complex is formed in which the copper is removed from the conductor surface and goes through a series of reactions in which copper sulfide is then formed on the copper surface. The information presented here suggests that DBDS degrades through cleaving of the disulfide linkage as the temperature increases in the oil resulting in the formation of mercaptans. Testing of some of these DBDS breakdown products has shown very corrosive results. Experiments at 110°C involving DBDS showed corrosion of the copper surface occurs in a relatively short period. Degradation of DBDS at temperatures lower than 110°C can also occur, and in experiments performed over the past several years corrosive sulfur attack on copper in oils with DBDS occurs at temperatures as low as 80°C in just over 60 days. Subsequent testing of that oil shows a reduction of DBDS during that time. Other researchers using a combination of copper and paper have detected the development of copper sulfide at 80°C in the Kraft paper insulation and on the copper surface. It might be that both processes, as well as others, occur simultaneously.
A review of the literature indicates that DBDS is usually found in concentrations ranging from 100 to 1000 mg/kg (ppm) in certain lubricating oils. DBDS is added to lubricating oils to protect against wear, reduce friction and increase oxidation stability. Through a method developed at Doble to detect DBDS, concentrations of 100 to 180 ppm have been found in new transformer oils, but this was only in a small percentage of the oil products tested. Whether DBDS is formed as a result of the refining process, added, or a combination of both is unknown. The chemical structure of DBDS is given in Figure 1.
The focus on DBDS is not without reasons. They are:
(1) Certain DBDS degradation by-products have been shown to be corrosive.
(2) Many oils involved in recent failures due to corrosive sulfur have had DBDS present.
(3) Trending analysis of DBDS in highly loaded transformers shows a reduction of its concentration with time.
(4) Laboratory analysis of oils spiked with DBDS has shown that as DBDS concentrations decline the corrosive nature of the oil increases, especially in sealed systems.
Results of Laboratory Experiments
Experiments were performed to elucidate the formation corrosive sulfur compounds in the oil from the degradation of DBDS. In these experiments DBDS was added to a white mineral oil in varying concentrations of 250, 125, 50 and 5 ppm (mg/kg). A white mineral oil has a very low total sulfur content measured at two-three ppm maximum. White oil that was used in the experiments was tested without the addition of DBDS and did not exhibit any signs of copper corrosion (ASTM D 1275B) or copper sulfide formation on the paper insulation by the Covered Conductor Deposition (CCD) test.
The white oil with 250 ppm of DBDS was tested using ASTM Test Method D 1275B (aging an abraded copper strip for 48 hours at 150°C). It was found to fail this test within 40 hours. In addition, the concentration of DBDS was determined before and after testing and found to have been reduced by more than 50 percent with a final concentration of 116 ppm.
Additional experiments were conducted to help in determining at what concentrations DBDS could still cause corrosion of the copper surface. Concentrations of DBDS in white oil were tested at 125, 50 and five ppm. The results of the testing are shown in Figures 2 (picture of corroded strips) and 3.
As shown in Figure 3, the difference in failure times between concentrations of 250, 125 and 50 were not that significant (40-48 hours). Even a concentration of five ppm corroded the copper strip over an extended period of time. This indicates that even small concentrations of DBDS may cause corrosive sulfur issues in electrical apparatus.
As a result of this experiment, a theory was put forth that DBDS was cleaved to the corresponding mercaptans (thiols) and other single-ring products. Further, four possible compounds could be formed of which benzyl mercaptan was the most likely. The chemical reaction is presented in Figure 4.
Benzyl mercaptan is very volatile and would not ordinarily be present in a newly refined transformer oil, as it would be easily removed. However, once produced, there is no escape in a well-sealed transformer. It is very oil soluble and is very reactive to copper and silver surfaces.
The same is not true in open conservator transformers where oxygen is present in higher concentrations. It was concluded that degradation of organic sulfur compounds involves an oxidative attack localized at the sulfur atom. As a result, benzyl mercaptan molecules are oxidized and some DBDS is actually reformed. Some of the benzyl mercaptan is likely lost through the free breathing nature of the conservator. Copper sulfide is formed but at least half or less than what would be formed in a sealed transformer. This chemical reaction is shown in Figure 5. It is most likely that some water is also formed, but the amount would be so minute in comparison to the water content already existing in the transformer, it would be indistinguishable.
A similar study using benzyl mercaptan instead of DBDS indicates that the reaction rate with the copper is very accelerated when compared to DBDS at 150°C. Refer to Figure 6.
Conclusions
It was determined that DBDS was found in certain transformer oils which were in transformers that had failed due to corrosive sulfur attack. Experiments showed that DBDS easily degraded into sulfur species that were highly reactive to copper and attacked the copper forming copper sulfide films. Theories were put forward indicating that at least one of the DBDS breakdown products is benzyl mercaptan and testing of this compound directly showed that it is highly corrosive. Chemical reactions showing the interaction of DBDS, benzyl mercaptan and components in the oil such as copper, hydrogen, and oxygen were provided, and a distinction was observed between sealed transformers and free breathing conservator transformers. This distinction is important as the failures of transformers due to corrosive sulfur attack with oils containing DBDS has been more prevalent in sealed transformers (nitrogen blanketed and systems with bladders) then those with free breathing conservator systems.

