6. Procedure
6.1 Determine d by Test Method D1298 if it is not otherwise available.
6.2 Determine L for the desired gas at T from Fig. 1, if it is shown.
6.3 Determine Lo from Table 1 for gases not shown in Fig. 1.
6.4 Calculate L for such gases by the equation
L = 0.300 exp [(0.639(700 - T)/T)ln 3.333Lo]
6.4.1 (Eq 1) is based on the assumption that all gases have L = 0.3 at 700 K (800°F). All the constants are empirical. The equation shall not be applied to such gases as methane or ethylene. When there is a difference, the result from Fig. 1 is to be preferred over that from Table 1.
6.5 The Ostwald coefficient L applies only to liquids of d = 0.85. To correct to other densities, use the following equation:
Lc = 7.70L(0.980 - d)
NOTE 1 - The constant 0.980 is based on the intermolecular volume of hydrocarbons as measured by compressibility and contraction on freezing. It is also the best empirical fit of the data. The use of this equation for very dense liquids is inadvisable, as the Ostwald coefficient becomes negative above d = 0.980. The constant 7.70 is also predictable from molecular theory, but the value used was determined empirically.
6.6 Calculate the Bunsen coefficient using the following equation:
B = 2697(p - pv)L/T
NOTE 2 - Fig. 2 shows the relations of the various solubility expressions to one another in chart form.
6.7 Calculate the solubility, which is expressed sometimes as parts per million by weight, using the following equation:
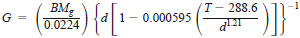
where:
0.0224 = molar volume at 273 K and 101.3 kPa in litres per mole, and 0.000595 and 1.21 = empirical constants for correcting d to specified T.
6.8 Calculate the solubility as the mol fraction by using the following equation:
X = 10(-6) G x Mt/Mg
(neglecting the moles of gas in the divisor)
6.9 Calculate the Henry's law constant as follows:
H = (p - pv)/X
6.10 Calculate the solubility coefficient for a mixture of gases as follows:
6.10.1 Calculate the individual Ostwald coefficients as described in 6.2 or 6.4, and combine them using the following equation:
Lm = (Lc1 x p1 + Lc2 x p2 . . . Lci x pi)/p
6.10.2 Calculate the Bunsen coefficient using the following equation:
Bm = 273pLm/T

