WATER-IMMISCIBLE ORGANIC SOLVENTS VOLATILE WITH STEAM
64. Scope
64.1 This test method covers the determination of water-immiscible organic solvents that are volatile with steam existing in a sample of sulfonated or sulfated oil, or both. Alcohol or other volatile compounds that are mutually soluble in water and solvents interfere in this test method.
65. Apparatus
65.1 General - The apparatus required consists of a glass distillation flask heated by suitable means and provided with a reflux condenser discharging into a trap and connected to the flask. The trap serves to collect and measure the condenser solvent and return the water to the flask. Except as specified in 65.2-65.5, the apparatus described in Section 5 shall be used.
65.2 Flask - A 300-mL glass distillation flask of the shortneck, round-bottom type, made of well-annealed glass.
65.3 Trap for Solvents Heavier than Water - For the determination of solvents heavier than water, the trap described in 5.1.4 shall be used.
65.4 Trap for Solvents Lighter than Water - For the determination of solvents lighter than water, the trap shall be made of well-annealed glass constructed in accordance with Fig. 4 and shall be graduated as shown from 0 to 5 mL in 0.1-mL divisions. The error of any indicated capacity shall not be greater than 0.05 mL.
65.5 Pycnometer - A pycnometer of 2-mL capacity shall be used for determining the specific gravity of solvents.
66. Reagents
66.1 Calcium Chloride (CaCl2).
66.2 Phenolphthalein, powder.
66.3 Sodium Carbonate (Na2CO3).
66.4 Sodium Hydroxide Solution (40 g/L) - Dissolve 40 g of sodium hydroxide (NaOH) in water and dilute to 1 L. Potassium hydroxide (KOH) may be substituted for NaOH.
67. Calibration
67.1 To calibrate the trap for solvents heavier than water, follow the procedure described in Section 7.
67.2 To calibrate the trap for solvents lighter than water (Fig. 4), close the plain arm of the trap with a one-hole rubber stopper through which is passed a short piece of glass tubing and connect the glass tube by means of rubber tubing to a separatory funnel (Note 16). Fill the funnel with dichloroethyl ether or other liquid heavier than, and nonmiscible with water, open the stopcock of the funnel, and carefully raise the funnel until enough of the liquid overflows into the return tube to reach the 0.3-mL mark on the graduated arm of the trap. From a standard pipet, introduce exactly 1 mL of water into the graduated arm, then add a drop of pine oil (to protect the surface from evaporation), and measure the volume of water in the arm. Raise the level of the solvent in the graduated arm by approximately 1 mL by running in more liquid from the funnel, and again measure the volume of water. Continue this procedure until the whole length of the graduated column of the trap has been calibrated.
NOTE 16 - Some traps are provided with an outlet and stopcock below the graduated arm. To calibrate such traps, the heavier-than-water liquid may very conveniently be introduced into the graduated column by connecting the separatory funnel directly to the outlet.
68. Procedure
68.1 Before using, clean the condenser and the receiving tube thoroughly with soap and warm water, rinse well, then treat with hot cleaning solution (a mixture of 10 mL of saturated potassium dichromate (K2Cr2O7) and 990 mL of sulfuric acid (H2SO4, sp gr 1.84)), and finally thoroughly wash and dry.
68.2 Transfer about 50 mL of water to the distillation flask. Take enough of the sample for analysis to yield about 4 mL of solvent (Note 17). Introduce the approximate quantity of the sample into a weighing bottle and make weighings from the bottle into the distillation flask, taking care that after removal of the sample no drops of oil are left on the outside of the bottle (Note 18). Rapidly neutralize the solution with NaOH solution (40 g/L) or KOH solution (40 g/L) until slightly alkaline to phenolphthalein. The phenolphthalein should preferably be added as a powder. Add to the mixture in the flask, 2 g of Na2CO3, enough CaCl2 to prevent foaming (3 to 5 g will usually suffice), and a few glass beads or pumice stone to prevent bumping. Fill the trap with water, using the trap for solvents heavier than water or the trap for solvents lighter than water, whichever is to be determined, and connect the apparatus.
NOTE 17 - In the presence of cresylic acid, use a sample large enough to yield about 2.5 mL of solvent. Before distillation is begun, add approximately 1.5 mL of pine oil, which should be weighed exactly, in order to reduce the specific gravity of the distillate.
NOTE 18 - In the case of highly volatile solvents, it is more accurate in weighing out the sample to remove the top of the weighing bottle and quickly to drop the bottle and its contents into the distillation flask.
68.3 Starting with the water at room temperature, heat the distillation flask at a rate such that the refluxing starts 7 to 10 min after the heat has been applied. Make a reading of the amount of solvent collected in the trap 2 h from the time the refluxing started. Make additional readings at the end of each subsequent 1-h period until the analysis is complete, that is, when the volume of solvent increases by not more than 0.1 mL in any two consecutive 1-h periods. Before making each reading, remove the source of heat from the distillation flask, and allow the flask to cool for 3 min (Note 19).
NOTE 19 - As an additional precaution, the distillation may be continued for another 15 to 30 min with the water in the condenser tube emptied.
NOTE 20 - When present, water-miscible solvents (such as alcohol) that are also miscible with the water-immiscible solvents will be found in the solvent layer. When there is an appreciable difference in the boiling points and specific gravities of the two types of solvents, they may be separated qualitatively by a fractional distillation and the specific gravities of the fractions determined. The amount of water-immiscible solvents may then be calculated from the specific gravities of the fractions and the specific gravity of the solvent layer.
In the case of pine oil or cresylic acid mixed with alcohol, the alcohol may be volatilized by heating over a hot plate, while stirring constantly, to a temperature of about 150°C, and then determining the specific gravity of the residue. The amount of water-immiscible solvent may now be calculated as in the previous procedure. This method may be applied to all such mixtures when there is considerable difference in boiling points and specific gravities and the specific gravity of one of the solvents is known.
When there is a difference in the specific gravities but not in the boiling points and the specific gravity of the water-miscible solvents is known, the water-immiscible solvent may be extracted with ether over water, the ether evaporated, and the specific gravity of the residue determined. In this procedure it is assumed that the boiling point of the water-immiscible solvent is considerably higher than that of ether. In all other cases it will be necessary to determine the water-immiscible solvent by quantitative fractional distillation or by chemical means.
68.4 When the analysis is complete, allow the distillate to stand for at least 40 min until it settles clear, and cools to about room temperature. Read the volume of the solvent in the trap. Immediately after reading, carefully remove about 2.5 mL of the solvent with a pipet and determine the specific gravity in the 2-mL pycnometer (Note 20). In the case of solvents heavier than water, separate the solvent from the water by means of a small separatory funnel.
68.5 For the analysis of samples containing solvents that are both heavier and lighter than water, make the distillation first with the trap for solvents heavier than water in place. Flush out all of the solvent floating on top of the water layer back into the distillation flask, substitute the trap for solvents lighter than water, and continue the distillation to completion.
69. Calculation
69.1 Calculate the percentage of water-immiscible volatile solvent by weight as follows:
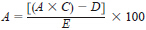
where:
A = percentage of water-immiscible volatile solvent by weight,
B = millilitres of solvents in traps,
C = specific gravity of solvents in traps,
D = correction for the addition of pine oil, if added, and
E = weight of sample, g.
70. Reproducibility of Results
70.1 Duplicate determinations by the same or different operators shall not differ from each other by more than 0.1 mL.

