ASTM D5808 Standard Test Method for Determining Organic Chloride in Aromatic Hydrocarbons and Related Chemicals by Microcoulometry
7. Apparatus
7.1 Pyrolysis Furnace, which can maintain a temperature sufficient to pyrolyze the organic matrix and convert all chlorine present in the sample to hydrogen chloride.
7.2 Pyrolysis Tube, made of quartz and constructed so that when a sample is volatilized in the front of the furnace, it is swept into the pyrolysis zone by an inert gas, where it combusts when in the presence of oxygen. The inlet end of the tube must have a sample inlet port with a septum through which the sample can be injected by syringe. The inlet end must also have side arms for the introduction of oxygen and inert carrier gas. The pyrolysis tube must be of ample volume, so that complete pyrolysis of the sample is ensured.
7.3 Titration Cell, containing a reference and sensor pair of electrodes and a generator anode/cathode pair of electrodes to maintain constant chloride ion concentration. An inlet from the pyrolysis tube and magnetic stirring is also required. (Warning - Excessive stirring speed will decouple the stirring bar and cause it to rise in the titration cell and possibly damage the electrodes. A slight vortex in the cell will be adequate.)
7.4 Microcoulometer, capable of measuring the potential of the sensing-reference electrode pair, and comparing this potential with a bias potential, and amplifying the difference to the working electrode pair to generate a current. The microcoulometer output voltage signal should be proportional to the generating current.
7.5 Automatic Boat Drive, having variable stops, such that the sample boat may be driven into the furnace, and stopped at various points as it enters the furnace.
7.6 Controller, with connections for the reference, working, and sensor electrodes. The controller is used for setting of operating parameters and integration of data.
7.7 Dehydration Tube, positioned at the end of the pyrolysis tube so that effluent gases are bubbled through a sulfuric acid solution, and water vapor is subsequently trapped, while all other gases are allowed to flow into the titration cell.
7.8 Gas-Tight Sampling Syringe, having a 50 µl capacity, capable of accurately delivering 10 to 40 µl of sample.
7.9 Quartz Boats.
8. Reagents and Materials
8.1 Purity of Reagents - Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
8.2 Purity of Water - Unless otherwise indicated, references to water shall be understood to mean reagent water conforming to Specification D1193, Type II or III.
8.3 Acetic Acid - Glacial acetic acid (CH3COOH).
8.4 Argon or Helium, 99.9 % minimum purity required as carrier gas.
8.5 Amidosulfonic Acid (H2NSO3H), minimum purity 99.3 - 100.3 %.
8.6 Sodium Acetate, anhydrous, (NaCH3CO2), fine granular.
8.7 Cell Electrolyte Solution - Dissolve 1.35 g sodium acetate (NaCH3CO2) in 850 mL of acetic acid (CH3COOH), and dilute to 1000 mL with water or follow manufacturer's recommendations.
NOTE 2 - Bulk quantities of the electrolyte should be stored in a dark bottle or in a dark place and be prepared fresh at least every two weeks.
8.8 Oxygen, 99.6 % minimum purity is required as the reactant gas.
8.9 Gas Regulators, two-stage gas regulators must be used for the reactant and carrier gas.
8.10 Potassium Nitrate (KNO3), fine granular.
8.11 Potassium Chloride (KCl), fine granular.
8.12 Potassium Sulfate (K2SO4), crystalline.
8.13 Working Electrode Solution (10 % KNO3) - Dissolve 50 g potassium nitrate (KNO3) in 500 mL of distilled water.
8.14 Inner Chamber Reference Electrode Solution (1 M KCl) - Dissolve 7.46 g potassium chloride (KCl) in 100 mL of distilled water.
8.15 Outer Chamber Reference Electrode Solution (1 M KNO3) - Dissolve 10.1 g potassium nitrate (KNO3) in 100 mL of distilled water.
8.16 Sodium Chloride (NaCl), fine granular.
8.17 Sodium Perchlorate (NaClO4), crystalline.
8.18 Sulfuric Acid, (sp gr 1.84), (H2SO4) concentrated.
8.19 2,4,6-Trichlorophenol (TCP) (C6H3OCl3), fine granular.
8.20 Solvent - The solvent of choice should be capable of dissolving the chloride sample. The solvent of choice should have a boiling point similar to the sample being analyzed. Suggested possibilities include, but not limited to, methanol, isooctane, toluene, and p-xylene.
8.21 Chloride Standard Stock Solution - Weigh accurately 0.093 g of 2,4,6-Trichlorophenol to 0.1 mg. Transfer to a 500-mL volumetric flask. Dilute to the mark with methanol.
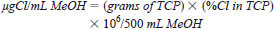
where:
TCP = 2,4,6, Trichlorophenol, and
MeOH = Methanol.
%Cl in TCP = 53.86

