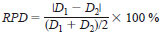10. Preparation of Apparatus
10.1 General Gas Chromatographic Conditions - The first temperature profile (12 min run time) is used for Tier I screening method for the presence of Aroclor. The longer second temperature profile (17 min run time) is used for Tier II to quantitate the Aroclors present, but may also be used for Tier I, if desired.
10.1.1 Rapid Screen Capilary Column Oven Temperature Profile (Tier I, 12 min run time):
Initial value: 130°C
Initial time: 2 min
Program rate: 20°C/min
Final value: 270°C
Final time: 3 min
Carrier gas: hydrogen
Head pressure: depend on DCB RT, (approximately 105 KPa (15 psi)) column, flow: 3.1-3.2 mL/min
Make-up gas: nitrogen or argon: methane
Make-up gas rate: approximately 65 mL/min.
Splitless mode
Purge off: 0 min
Purge on: 1.0 min
Purge vent: 2.5 mL/min
Split vent: 50 mL/min
Sample injection: 2.0 uL
Injector inlet system: 250°C
Detector: 315°C
10.1.2 Quantitation Capillary Column Oven Temperature Profile (Tier II, 17 min run time; may also be used for Tier I analysis:
Initial value: 125°C
Initial time: 3 min
Level I
Program rate: 12°C/min
Final value: 270°C
Final time: 2 min
Carrier gas: hydrogen
Head pressure: Depend on DCB RT (approximately, 105 KPa (15 psi))
Column flow: 3.1 mL/min (approximately at 270°C)
Make-up gas: nitrogen
Make-up gas rate: approximately 65 mL/min
Splitless mode
Purge off: 0 min
Purge on: 1.0 min
Purge rate: 50 mL/min
Sample injection: 2.0 uL
Injector inlet system: 250°C
Detector: 315°C
11. Calibration and Standardization
11.1 Calibration:
11.1.1 Tier 1 - Screening Method - Aroclors are multi-peak chemical mixtures that have very unique identification patterns. All Aroclors shall be run individually or in mixtures at 0.1 ug/mL on each channel performing screening to produce reference patterns. It is important to note that some of these patterns have the same constituents and that some Aroclors are quantitated using the same peaks (such as Aroclors 1016 and 1232 or 1242). When screening for Aroclors, a visual determination is made by the following key items:
11.1.1.1 Aroclor pattern - (a) same singlets, doublets, and triplets present in the reference chromatograms, and (b) same relative peak heights between peaks in the sample chromatogram and the reference chromatogram.
11.1.1.2 Retention time shifts should be very consistent between the standard and the sample peaks.
11.1.1.3 All samples in which an Aroclor is detected (using Tier I) require a judgment concerning the amount. The recognized Aroclor pattern shall be compared to the IPS (0.01 ug/mL or 0.1 ug/mL). If the overall level of the suspected Aroclor pattern is equal to or greater than overall level of the IPS pattern, then Tier II analysis may be used to quantitate the sample. If multiple Aroclors are suspected, a Tier II analysis may be run to help resolve the mixture.
11.1.1.4 Recovery control limits for the surrogate are 40 to 150 % recovered. If the recovery is outside of these limits, see Annex A1.
11.1.2 Tier I Calibration Check - An instrument performance standard (IPS) at 0.01 ug/mL of Aroclor 1016 and 1260 is used to check the instrument sensitivity once a day or every 20 samples, whichever is more frequent (typically laboratories using ten samples compositing shall use the 0.01 ug/mL standard to achieve a detection limit of 5 ug/mL of Aroclor in any individual sample). Sample results will be compared qualitatively with the daily IPS. (See the Calculation section 13).
11.1.2.1 Tabulate the sum of the areas or the data system calculated amount of the five major peaks for each of the Aroclors 1016 ad 1260 in the instrument performance standard. The response shall be within 50 % of the initial response. Initial response shall be established by averaging the response of a minimum of five injections of the instrument performance standard (IPS). If the limit is exceeded, new limits may need to be established.
11.1.2.2 Likewise, the expected response for the surrogate, if used, is established by averaging the areas of DCB in the five initial IPS analyses.
11.1.2.3 The surrogate also may be used for retention time control. It is recommended that column flow be adjusted so DCB elutes between 10.5 to 11.5 min using the 12 min GC program. (This will typically require a column head pressure of 105 to 112 kPa.) (Alternatively, the retention time should be 15 to 16.5 min using the 17 min program.)
11.1.3 Tier 2 - Quantitative Method - The GC data system must be calibrated for both Aroclors 1016 and 1260, using five peaks for each Aroclor. [For example, when using an integrator, divide the standard amount by the number of peaks being used. Using five peaks on a 0.5 ug/mL standard would assign 0.1 ug/mL to each peak. This will allow for a calibration table to be made, yielding response factors for each peak at the five levels of calibration. Set up a calibration table in the method file of the integrator or data system that is to be used. Calculate an average response factor for each of five peaks for both Aroclors. Calculate the standard deviation of the average response factor for each peak of the Aroclor using the following calculation.
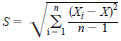
where:
S = standard deviation,
Xi = each observed value,
X = the arithmetic mean of observed values, and
n = total number of calibration points.
11.1.3.1 Calculate the percent relative standard deviations (% RSDs) for the response factors of the calibrated peaks for each Aroclor from the formula below. The acceptance criteria for the % RSD for each Aroclor is not more than 20 %. If the average % RSD is greater than 20 % for either Aroclor, then linearity over the desired calibration range for that instrument has not been demonstrated.
NOTE 8 - The % RSD is 100 % multiplied by the result of Eq 3 (s) divided by the arithmetic mean ( X).
11.1.3.2 When samples are to be analyzed, instrument control is verified by analyzing the CCS and the percent difference (% D) is calculated. The acceptance criteria is within +30 % for each AROCLOR in the CCS (1016 and 1260).
11.1.3.3 If either Aroclor 1016 or 1260 is out of control for the daily CCS, corrective action shall be taken and a CCS reanalyzed. If corrective action does not correct the problem, then a new five point calibration curve shall be created. Percent difference (% D)
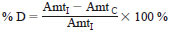
where:
Amt I = amount in standard, and
AmtC = calculated amount from current CCS.
11.1.3.4 Calibration for Aroclors other than Aroclor 1016 and Aroclor 1260 will be performed by analyzing standards at the concentration representing the midpoint of the calibration range selected. For example, if calibration is desired over the range of 0.02 ug/mL to 0.5 ug/mL, then the 0.1 ug/mL standards shall be used for calibration. Therefore, a five point calibration shall be performed for Aroclors 1016 and 1260 and a one-point calibration shall be performed for all remaining Aroclors.
11.1.3.5 After the linearity of the system has been demonstrated, and each of the remaining Aroclors has been analyzed using middle level concentration, recalibration will be required only when the calibration check standard criteria is met. Old calibration curves may not be used again, other than to review data generated using those calibration curves.
11.2 Standardization:
11.2.1 Surrogate Recovery - Recovery control limits for the surrogate are 40 to 150 % recovered.
11.2.1.1 If the recovery is outside of these limits, see Annex A1.
11.2.2 Method Blank - For every 20 samples or batch, whichever is more frequent, a method blank shall be prepared by processing the extraction solvent (with surrogate, if used) through the same clean-up as that used for the samples. This is to detect possible contamination picked up during the sample clean-up process.
NOTE 9 - A batch is the group of samples prepared at the same time. A batch may not exceed 20 samples.
11.2.3 Calibration Check Standard (CCS) (Tier II only) - A 0.1 ug/mL standard (or 0.2 ug/mL) obtained from a source separate from the intermediate standard and containing Aroclors 1016 and 1260 is the CCS which is used to verify the validity of the five-point calibration curve. The calculated results for the CCS shall agree with the current calibration curve to within +/-30 % percent difference (% D). If the CCS results indicate that the calibration is outside control limits, and routine maintenance does not correct the problem, then the GC/ECD must be recalibrated.
11.2.4 Matrix Spike (MS) Samples (Tier II only) - For every batch or twenty samples, whichever is more frequent, a sample requiring Tier II analysis shall be selected in an unbiased manner and spiked with Aroclor 1268. These results shall be documented, with an example shown in Appendix X2.
11.2.4.1 1.0 mL of 50 ug/mL of Aroclor 1268 (25 ug/mL, if working at lower calibration range) is added to the sample chosen for spiking. Matrix spiked sample recovery limits are from 60 to 140 %, providing any Aroclor present in the sample before spiking does not exceed five times the spike level.
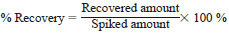
11.2.5 Matrix Spike Duplicate (MSD) Sample (Tier II only) - Every batch or 20 samples, whichever is more frequent, precision data is generated using a matrix spike duplicate. Acceptance criteria is 20 % relative percent difference (RPD) for the duplicate analyses.
11.2.5.1 RPD is calculated from the absolute difference between duplicate percent recovery results D 1 and D2 divided by the mean value of the duplicates.