METHOD A - COLOR INDICATOR TITRATION
6. Apparatus
6.1 Erlenmeyer Flask and Condenser - An Erlenmeyer flask, 250 or 300-mL capacity, alkali-resistant (see Note 2) and (Warning - Causes severe burns; a recognized carcinogen; strong oxidizer - contact with other material can cause fire; hygroscopic), to which is attached a straight or mushroom-type reflux condenser. The straight-type condenser is fitted to the flask with a ground-glass joint; the mushroom-type condenser must fit loosely to permit venting of the flask. Water reflux condensers can also be used instead of air condensers.
NOTE 2 - Do not use scratched or etched Erlenmeyer flasks because KOH will react with them. The glassware shall be chemically clean. It is recommended that flasks be cleaned with chromic acid cleaning solution (Alternatively, Nochromix or similar products can be used.)
6.2 Hot Plate - A suitable hot plate heated by either electricity or steam. (Warning - Thermal hazard; in addition to other precautions, avoid contact with exposed skin.)
7. Reagents
7.1 Purity of Reagents - Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.
NOTE 3 - Commercially available reagents may be used in place of laboratory preparations, provided they meet the specifications outlined.
7.2 Purity of Water - Unless otherwise indicated, references to water shall be understood to mean reagent water as defined by Type III in Specification D1193.
7.3 Alcohol - 95 % ethanol (Warning - Flammable. Denatured - Cannot be made nontoxic) (see Note 4) and (Warning - Flammable) or 95 % ethanol to which has been added 10 volume % of methanol (see Note 4 and Note 5) or absolute alcohol.
NOTE 4 - It has been found that 99 % 2-propanol (isopropyl alcohol) can be substituted for the purified ethanol with entirely satisfactory results. This substitution is not permissible, however, in referee tests.
NOTE 5 - This composition is available under the name of "U.S. Department of Treasury Specially Denatured Formula 30 (Regulation No. 3-1938)". Formula 3A plus 5 % methanol is an equivalent.
7.4 Aqueous Hydrochloric Acid Standard Solution (0.5 M) - Standardize to detect molarity changes of 0.0005 by titrating with standard alcoholic KOH solution (see 7.8 and Note 6).
NOTE 6 - Where saponification numbers below one are expected, better precision can be obtained by substituting 0.1 M KOH solution and HCl for the 0.5 M reagents in Sections 7, 8, 10, 17, and 19.
7.5 Butanone (Methyl Ethyl Ketone), technical grade. Store in dark or brown bottles. (Warning - See 4.1.)
7.6 Naphtha, (Warning - Extremely flammable. Harmful if inhaled. Vapors can cause flash fire.) ASTM Precipitation Grade (or Petroleum Spirit-60/80 or hexanes) (Warning - Combustible. Vapor harmful.) Petroleum spirit shall conform to the current IP 136.
7.7 Phenolphthalein Solution, Neutralized - Dissolve 1.0 more or less 0.1 g of phenolphthalein in 100 mL of alcohol (see 7.3). Neutralize to faint pink color with dilute (0.1 M) alcoholic KOH solution.
7.8 Alcoholic Potassium Hydroxide Standard Solution (0.5 M) - Prepare approximately 0.5 M solution by dissolving KOH in the alcohol specified in 7.3. Allow the solution to settle in a dark place. Filter the solution, and allow to stand for 24 h before using.
7.8.1 Alternatively prepare 0.5 or 0.1 M alcoholic KOH by mixing a commercially available KOH ampule (which is carbonate free) with 95 % alcohol. Using this type solution gives consistent blanks and does not give multiple breaks (see Note 7).
NOTE 7 - Because of the relatively large coefficient of cubic expansion of organic liquids such as 2-propanol (isopropyl alcohol), the standard alcoholic solution has to be standardized at temperatures close to those employed in the titrations of samples.
7.8.2 The KOH solutions shall be standardized by titrating with standard potassium hydrogen phthalate solution (see 7.9 and Note 7).
7.9 Potassium Hydrogen Phthalate - (C8H5KO4) 0.1 M Standard Solution - Weigh 2.0422 more or less 0.0002 g of potassium hydrogen phthalate that has been dried at 110 more or less 5°C to a constant weight into a 100-mL volumetric flask. Dissolve in reagent water. Some heating may be necessary to dissolve the solid. Dilute to 100 mL with distilled or deionized water, after the solution has cooled.
7.10 Stoddard Solvent, technical grade. (Warning - Extremely flammable. Harmful if inhaled.)
7.11 Xylene, reagent grade. (Warning - Extremely flammable. Harmful if inhaled.)
8. Blank Determinations
8.1 Perform a blank determination concurrently with each set (see Note 8) (one or more) of samples as follows: measure accurately from a buret or volumetric pipet (see Note 9) into the Erlenmeyer flask 25 more or less 0.03 mL of alcoholic KOH solution and 25 more or less 1 mL of butanone (methylethyl-ketone) or one of the alternative solvents. Connect the condenser to the flask, and heat for the same amount of time as that used for the sample after refluxing begins. (Warning - The reflux condenser should be clamped securely to prevent it from tipping over onto the hot plate with possible breakage of glassware. See also Note 10.) Immediately add 50 mL of ASTM precipitation naphtha (Warning - See 7.6, also Note 11 and Note 12) by cautiously pouring the naphtha down the condenser (disconnect condenser if mushroom-type is used), and titrate the blank while hot, without reheating, with 0.5 M hydrochloric acid (HCl) using three drops of neutralized phenolphthalein indicator solution.
NOTE 8 - Run blank determinations in duplicate on samples requiring the highest accuracy. The precision data are based on duplicate blank determinations. A single blank is sufficient for routine work.
NOTE 9 - If a volumetric pipet is used to measure the alcoholic KOH solution, wait 30 s after delivery to allow for complete drainage.
NOTE 10 - Although standard procedure requires 30 min of reflux, some fats are readily saponified and complete saponification takes place within 10 min. On the other hand, difficult saponifiable materials require more than 2 h. Neither the shortened period nor the longer period should be used except by mutual consent of the interested parties.
NOTE 11 - Pouring 50 mL of naphtha down the condenser at the end of the saponification not only rinses the condenser but also cools the reaction mixture.
NOTE 12 - In the case of insulating oils, the addition of ASTM precipitation naphtha or petroleum spirit is not necessary.
8.2 After the indicator color has been discharged, add, dropwise, more indicator solution. If this addition of indicator restores the color, continue the titration, making further dropwise additions of indicator, if necessary, until the end point is reached (Note 13). The end point is reached when the indicator color is completely discharged and does not immediately reappear upon further dropwise addition of the indicator solution. Record as V1 in 11.1.
NOTE 13 - Avoid emulsification of titration mixture, but ensure phase contact by swirling the flask vigorously as the end point is approached.
9. Sample
9.1 Using Practice D4057 (manual sampling) or Practice D4177 (automatic sampling) as a guideline for obtaining a representative sample, make sure that the portion of the sample to be tested appears homogenous. Choose the size of the sample so that the back-titration volume is from 40 to 80 % of the blank, but do not exceed a 20-g sample weight (see Note 14).
NOTE 14 - The following sample sizes are suggested:
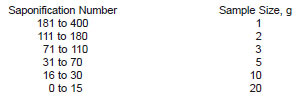
10. Procedure
10.1 Weigh the specimen to the nearest 0.01 g (record as W in 11.1), such as by difference, from a small beaker into the Erlenmeyer flask. Add 25 more or less 1 mL of butanone or one of the alternative solvents (Warning - See 4.1), followed by 25 more or less 0.03 mL of alcoholic KOH solution (Warning - See 7.3) measured accurately from a buret or volumetric pipet (see Note 6).
10.2 Dissolve the difficult to dissolve samples, such as lubricants and additives, first in 15 to 25 mL of Stoddard Solvent (Warning - See 7.10) or xylene (Warning - See 7.11) before adding butanone (Warning - See 4.1).
10.3 Connect the condenser to the flask and heat for 30 min after refluxing begins (see Note 10). Immediately add 50 mL of ASTM precipitation naphtha (Warning - Do not pour naphtha while the flask is on the hot plate) and (see 7.6) by cautiously pouring the naphtha down the condenser (see Note 11) (disconnect condenser if mushroom-type before adding the naphtha).
10.4 Titrate the solution while hot (without reheating) with 0.5 M HCl using three drops of neutralized phenolphthalein indicator solution (Warning - See 7.3). When the indicator color is discharged, add, dropwise, more indicator solution. If this addition of indicator restores the color, continue the titration, making further dropwise additions of indicator, if necessary, until the end point is reached (Note 13). The end point is reached when the indicator color is completely discharged and does not immediately reappear upon further dropwise addition of the indicator solution. (Record as V2 in 11.1.) When testing waxes, it may be necessary to reheat the solution during titration to prevent solidification of the sample.
11. Calculation
11.1 Calculate the saponification number, A, as follows:
A = 56.1 M(V1 - V2)/W
where:
M = molarity of the hydrochloric acid,
V1 = volume of acid used in titrating the blank, mL,
V2 = volume of acid used in titrating the sample, mL,
W = sample, g, and
56.1 = molecular weight of KOH.
12. Report
12.1 For saponification numbers of less than 50, report the saponification number to the nearest 0.5 mg KOH/g sample.
12.2 For saponification number of 50 or more, report to the nearest whole number.
12.3 For electrical insulating oils, report the values to the nearest 0.1 mg KOH/g sample.
12.4 Report the saponification numbers as obtained by Test Methods D94, Method A.
13. Precision and Bias
13.1 Precision - The data shown in Fig. 1 shall be used for judging the acceptability of results (95 % probability) (see Note 15).
NOTE 15 - No precision intervals can be given for highly colored new or used oils, or for oils that produce dark-colored solutions upon saponification, as color can interfere with the detection of the end point of the titration. In such cases, potentiometric titration (Method B) can be used.
13.1.1 Repeatability - The difference between two test results, obtained by the same operator with the same apparatus under constant operating conditions on identical test material, would in the long run, in the normal and correct operation of the test method, exceed the Fig. 1 values only in one case in twenty.
13.1.2 Reproducibility - The difference between two single and independent results obtained by different operators working in different laboratories on identical test materials would, in the long run, in the normal and correct operation of the test method, exceed the Fig. 1 values only in one case in twenty.
13.2 Bias - This is an empirical test method, and there are no accepted reference materials that can be compared; hence, bias cannot be determined.

