7. Sensitivity (Response)
7.1 Sensitivity (response) of the FID is the signal output per unit mass of a test substance in the carrier gas, in accordance with the following relationship:
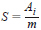
where:
S = sensitivity (response), A•s/g,
Ai = integrated peak area, A•s, and
m = mass of the test substance in the carrier gas, g.
7.2 Test Conditions:
7.2.1 Normal butane is the preferred standard test substance.
7.2.2 The measurement must be made within the linear range of the detector.
7.2.3 The measurement must be made at a signal level at least 200 times greater than the noise level.
7.2.4 The test substance and the conditions under which the detector sensitivity is measured must be stated. This will include, but not necessarily be limited to, the following:
7.2.4.1 Type of detector,
7.2.4.2 Detector geometry (for example, electrode to which bias is applied),
7.2.4.3 Carrier gas,
7.2.4.4 Carrier gas flow rate (corrected to detector temperature and fluid presssure),
7.2.4.5 Make-up gas,
7.2.4.6 Make-up gas flow rate,
7.2.4.7 Detector temperature,
7.2.4.8 Detector polarizing voltage,
7.2.4.9 Hydrogen flow rate,
7.2.4.10 Air or oxygen flow rate,
7.2.4.11 Method of measurement, and
7.2.4.12 Electrometer range setting.
7.3 Methods of Measurement:
7.3.1 Sensitivity may be measured by any of three methods:
7.3.1.1 Experimental decay with exponential dilution flask (7) (see 7.4).
7.3.1.2 Utilizing the permeation device (8) under steady-state conditions (see 7.5).
7.3.1.3 Utilizing Young’s apparatus (9) under dynamic conditions (see 7.6).
7.3.2 Calculation of FID sensitivity by utilizing actual chromatograms is not preferred because in such a case the amount of test substance at the detector may not be the same as that introduced.
7.4 Exponential Dilution Method:
7.4.1 Purge a mixing vessel of known volume fitted with a magnetically driven stirrer with the carrier gas at a known rate. The effluent from the flask is delivered directly to the detector. Introduce a measured quantity of the test substance into the flask to give an initial concentration, Co, of the test substance in the carrier gas, and simultaneously start a timer.
7.4.2 Calculate the concentration of the test substance in the carrier gas at the outlet of the flask at any time as follows (see Annex A1):
Cf = Co exp [-Fft/Vf]
where:
Cf = concentration of the test substance at time t after introduction into the flask, g/mL,
Co = initial concentration of the test compound introduced into the flask, g/mL,
Ff = carrier gas flow rate, corrected to flask temperature (see Annex A1), mL/min,
t = time, min, and
Vf = volume of flask, mL.
7.4.3 Calculate the sensitivity of the detector at any concentration as follows:
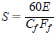
where:
S = sensitivity, A•s/g,
E = detector signal, A,
Cf = concentration of the test substance at time, t, after introducton into the flask, g/mL, and
Ff = carrier gas flow rate, corrected to flask temperature (see Annex A1), mL/min.
NOTE 2 - This method is subject to errors due to inaccuracies in measuring the flow rate and flask volume. An error of 1 % in the measurement of either variable will propagate to 2 % over two decades in concentration and to 6 % over six decades. Therefore, this method should not be used for concentration ranges of more than two decades over a single run.
NOTE 3 - A temperature difference of 1 C between flask and flow-measuring apparatus will, if uncompensated, introduce an error of 1/3 % into the flow rate.
NOTE 4 - Extreme care should be taken to avoid unswept volumes between the flask and the detector, as these will introduce additional errors into the calculations.
NOTE 5 - Flask volumes between 100 and 500 mL have been found the most convenient. Larger volumes should be avoided due to difficulties in obtaining efficient mixing and likelihood of temperature gradients.
NOTE 6 - This method may not be used with supercritical-fluid mobile phases unless the flask is specifically designed and rated for the pressure in use.
7.5 Method Utilizing Permeation Devices:
7.5.1 Permeation devices consist of a volatile liquid enclosed in a container with a permeable wall. They provide low concentrations of vapor by diffusion of the vapor through the permeable surface. The rate of diffusion for a given permeation device is dependent only on the temperature. The weight loss over a period of time is carefully and accurately determined; thus, these devices have been proposed as primary standards.
7.5.2 Accurately known permeation rates can be prepared by passing a gas over the previously calibrated permeation device at constant temperature. Knowing this permeation rate, Rt, the sensitivity of the detector can be obtained from the following equation:
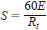
where:
S = sensitivity, A•s/g,
E = detector signal, A, and
Rt = permeation rate of a test substance from the permeation device, g/min.
NOTE 7 - Permeation devices are suitable only for preparing relatively low concentrations in the part-per-million range. In addition, only a limited range of linearity can be explored because it is experimentally difficult to vary the permeation rate over an extended range. Thus, for detectors of relatively low sensitivity or of higher noise levels, this method may not satisfy the criteria given in 4.2.3, which requires that the signal be at least 200 times greater than the noise level. A further limitation in the use of permeation devices is the relatively slow equilibration of the permeation rate, coupled with the life expectancy of a typical device which is on the order of a few months.
NOTE 8 - This method may not be used with supercritical-fluid mobile phase. SC-CO2 would adversly affect the permeation tube by either extracting the polymer or swelling the tube, resulting in a potential safety hazard.
7.6 Dynamic Method:
7.6.1 In this method, inject a known quantity of test substance into the flowing carrier gas stream. A length of empty tubing or an empty high-pressure cell between the sample injection point and the detector permits the band to spread and be detected as a Gaussian band. Then integrate the detector signal by any suitable method. This method has the advantage that no special equipment or devices are required other than conventional chromatographic hardware.
7.6.2 As an alternative to 7.6.1, an actual chromatogram may be generated by substituting a column for the length of empty tubing. This approach is not preferred because it is common for the sample to have adverse interaction with the column. These problems can be minimized by using an inert stable liquid phase loaded sufficiently to overcome support adsorption effects. Likewise a nonpolar sample will minimize these adverse interactions. For example, a column prepared with OV101 on Chromosorb G with a n-octane sample should best ensure that the entire sample introduced will reach the detector.
7.6.3 Calculate the sensitivity of the detector from the peak area and the mass injected in accordance with 7.1.
NOTE 9 - Care should be taken that the peak obtained is sufficiently wide so the accuracy of the integration is not limited by the response time of the recording device.
NOTE 10 - The approach given here should be used with caution as it has not been applied over a wide concentration range.

