IEC 60422 Mineral Insulating Oils in Electrical Equipment - Supervision and Maintenance Guidance
5 Oil tests and their significance
5.1 General
A large number of tests can be applied to mineral insulating oils in electrical equipment. The tests listed in Table 1 and discussed in 5.2 to 5.19 are considered sufficient to determine whether the condition of the oil is adequate for continued operation and to suggest the type of corrective action required, where applicable. The tests are not listed in order of priority.
5.2 Colour and appearance
The colour of an insulating oil is determined in transmitted light and is expressed by a numerical value based on comparison with a series of colour standards. It is not a critical property, but it may be useful for comparative evaluation. A rapidly increasing or a high colour number may be an indication of oil degradation or contamination.
Besides colour, the appearance of oil may show cloudiness or sediment, which may indicate the presence of free water, insoluble sludge, carbon particles, fibres, dust, or other contaminants.
5.3 Breakdown voltage
Breakdown voltage is a measure of the ability of oil to withstand electric stress and has primary importance for the safe operation of electrical equipment. It is strongly dependent on the sampling temperature (5.4.3 and 5.4.4).
Dry and clean oil exhibits an inherently high breakdown voltage. Free water and solid particles, the latter particularly in combination with high levels of dissolved water, tend to migrate to regions of high electric stress and reduce breakdown voltage dramatically. The measurement of breakdown voltage, therefore, serves primarily to indicate the presence of contaminants such as water or particles. A low value of breakdown voltage can indicate that one or more of these are present. However, a high breakdown voltage does not necessarily indicate the absence of all contaminants.
The values of breakdown voltage are only significant when the oil has been sampled at the operating temperature of the transformer. Samples taken at < 20 °C may give an optimistic view of the state of the transformer when analysed at room temperature. The breakdown voltage of spare units that have been long out of service and are again energized should be monitored more often until the transformer has reached a steady state.
5.4 Water content
5.4.1 General
Depending on the amount of water, the temperature of the insulating system and the status of the oil, the water content of insulating oils influences
• the breakdown voltage of the oil,
• the solid insulation,
• the ageing tendency of the liquid and solid insulation.
The water content in the liquid and solid insulation thus has a significant impact on the actual operating conditions and the lifetime of the transformer.
There are two main sources of water increase in transformer insulation:
• ingress of moisture from the atmosphere;
• degradation of insulation.
Water is transferred in oil filled electrical equipment by the insulating liquid. Water is present in oil in a dissolved form and may also be present as a hydrate adsorbed by polar ageing products (bonded water). Particles, such as cellulose fibres may bind some water.
5.4.2 Water in oil
The solubility of water in oil (Ws), given in mg/kg, depends on the condition of the oil, the temperature and type of oil. The absolute water content (Wabs) is independent of the temperature, type and condition of the oil and the result is given in mg/kg. Wabs can be measured according to IEC 60814. The relative water content (Wrel) is defined by the ratio Wabs/Ws and the result is given in per cent. The relative water content can be evaluated by use of a suitable method such as that in BS 6522 or on-line by means of capacitive sensors. Water solubility (Ws) should be determined at the same temperature as that of the oil sample when taken. By way of a guide, the condition of cellulosic insulation in relation to oil percentage saturation is given in Table A.1.
At water contents in oil above the saturation level, i.e. when Wabs > Ws (or Wrel > 100 %), the excess water cannot remain dissolved and free water may be seen in the form of cloudiness or droplets.
Usually, the temperature is determined directly in the oil stream of the sample taken. In cases where top oil indicator readings or corrections for ONAN (natural oil or natural air) or OFAF (forced oil or forced air) cooling mode are used, this should be explicitly noted.
The water content in oil is directly proportional to the relative water concentration (relative saturation) up to the saturation level. The temperature dependence of the solubility of water in oil (WS) is expressed by:
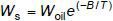
where T is the temperature of the oil at the point of sampling in Kelvin and W 0il and B are constants that are similar for many transformer oils but may be different for some products, mainly due to differences in aromatic content. Where present, some free water may transfer into dissolved water at elevated temperatures.
As oils become very oxidized with increasing amounts of polar ageing by-products, their water solubility characteristics, which are also dependent on the type of the oil, also increase. The solubility of water in very aged oils may be much higher than that in unused oils (Figure 1). Each oil should be considered separately and no universal formula is available.
5.4.3 Water content in the oil/paper-system
Transformers are dried during the manufacturing process until measurements or standard practices yield a moisture content in the cellulosic insulation of less than 0,5 % to 1,0 % depending upon purchaser's and manufacturer's requirements. After the initial drying, the moisture content of the insulation system increases depending on the environmental and/or operating conditions.
In a transformer, the total mass of water is distributed between the paper and the oil such that the bulk of the water is in the paper. Small changes in temperature significantly change the dissolved water content of the oil but only slightly change the water content of the paper.
When oil in a transformer is operating at a constant, relatively elevated temperature for a long period, thermodynamic equilibrium between water absorbed by cellulose and water dissolved in oil is closely approached. This equilibrium is temperature dependent so that at elevated temperatures more water diffuses from the paper into the oil. However, if the oil temperature is not high enough, such equilibrium is not reached because of the lower rate of diffusion of water to the oil from the cellulose insulation.
The determination of the water content in the paper of a transformer by the measurement of the water in oil has been frequently described, but practical results are often not in line with the theoretical predictions. The drying process of the paper may not take out as much water as calculated.
All calculations and correlations of the water content in oil and the water content in the oil/paper-system depend on the equilibrium state between the insulating oil and the oil/paper-system and vice versa. The equilibrium is influenced by many factors, such as the difference in the temperature between oil and the cellulose/oil-system. The calculation of the water content of the paper/pressboard by determination of the water in the oil has been examined in several studies and publications (see Annex A).
5.4.4 Interpretation of results
Breakdown voltage and water content are strongly interrelated. Both of them are temperature dependent, therefore it is most informative to measure them at different transformer temperatures in order to obtain a reliable assessment of humidity in the combined oil-paper insulation system. The interpretation of water content in oil is strongly related to the sampling temperature determined by measuring the temperature directly in the oil stream. In cases where the top oil temperature indicator (OTI) temperature corrections for ONAN or OFAF cooling mode are used, this should be explicitly noted.
For transformers with a relatively steady load, a normalizing calculation of the water content for 20 °C may be helpful for trending. The procedure is described in Annex A.
5.5 Acidity
The acidity (neutralization value) of oil is a measure of the acidic constituents or contaminants in the oil.
The acidity of a used oil is due to the formation of acidic oxidation products. Acids and other oxidation products will, in conjunction with water and solid contaminants, affect the dielectric and other properties of the oil. Acids have an impact on the degradation of cellulosic materials and may also be responsible for the corrosion of metal parts in a transformer.
The rate of increase of acidity of oil in service is a good indicator of the ageing rate. The acidity level is used as a general guide for determining when the oil should be replaced or reclaimed.
Generally, inhibited oil should show no significant increase in acidity from its original value provided that the inhibitor is present in sufficient amount.
5.6 Dielectric dissipation factor (DDF) and resistivity
These parameters are very sensitive to the presence of soluble polar contaminants, ageing products or colloids in the oil. Changes in the levels of the contaminants can be monitored by measurement of these parameters even when contamination is so slight as to be near the limit of chemical detection.
Acceptable limits for these parameters depend largely upon the type of equipment. However, high values of DDF, or low values of resistivity, may deleteriously affect the dielectric losses and/or the insulation resistance of the electrical equipment.
There is generally a relationship between DDF and resistivity, with resistivity decreasing as DDF increases. It is normally not necessary to conduct both tests on the same oil and generally DDF is found to be the more common test. Resistivity and DDF are temperature and moisture dependent and Figure 2 illustrates typical changes of resistivity with temperature and moisture for insulating oils that are virtually free from solid contamination.
Useful additional information can be obtained by measuring resistivity or DDF at both ambient temperature and a higher temperature such as 90 °C.
In the case of very high voltage (VHV) and ultra high voltage (UHV) instrument transformers, special attention shall be paid to DDF as it has been reported that a higher value of DDF may lead to thermal runaway leading to failure.
Oils classified as "good" (see 9.4) will have characteristics similar to curves A and B in Figure 2 and will result in satisfactory test results being obtained at both the higher and lower temperatures.
Oils classified as "poor" (see 9.4) will have characteristics similar to curve C and will result in a satisfactory test result at 90 °C coupled with an unsatisfactory value at the lower temperature. This is an indication of the presence of water or degradation/deterioration products precipitable in the cold without any significant amount of chemical degradation or general contamination. Unsatisfactory results at both temperatures indicate a greater extent of contamination and that it may not be possible to restore the oil to a satisfactory condition by reconditioning.
The measurement of resistivity is also considered to be of value for monitoring oils in service, as it has been shown to be reasonably proportional to oxidation acids and to be affected by undesirable contaminants such as metal salts and water. Other compounds present in used oils, which can affect resistivity, include aldehydes, ketones and alcohols. An increase in temperature reduces the resistivity, as does water when precipitated at low temperature due to the saturation point being reached.
It has been observed in instrument transformers that some types of oil may experience a huge increase in DDF after a very short oxidation time, leading to failure of the equipment. It is therefore recommended to measure the DDF of the unused oil after subjecting it to a short oxidation period according to IEC 61125:1992, Method C to verify that the oil is suitable for this application.
5.7 Inhibitor content and oxidation stability
5.7.1 Oxidation stability
The ability of unused mineral insulating oil to withstand oxidation under thermal stress and in the presence of oxygen and a copper catalyst is called oxidation stability. It gives general information about the life expectancy of the oil under service conditions in electrical equipment. The property is defined as resistance to formation of acidic compounds, sludge and compounds influencing the dielectric dissipation factor (DDF) under given conditions. For oils complying with IEC 60296, these conditions are detailed in IEC 61125:1992, Method C and the limits of acceptable performance in IEC 60296.
The property depends mainly on the refining process and how it is applied to a given feedstock. Refined mineral oils contain, to a varying degree, natural compounds acting as oxidation inhibitors. These are known as natural antioxidants. Oils containing only natural antioxidants are designated as uninhibited oils.
Synthetic oxidation inhibitors can be added to enhance the oxidation stability. In transformer oils, mainly the phenolic type is used and the common and generally accepted compounds are 2,6-di-tert-butyl-paracresol (DBPC) and 2,6-di-tert-butyl-phenol (DBP). The efficacy of added inhibitors will vary with the chemical composition of the base oil.
To determine the oxidation stability, tests specified in IEC 61125:1992 Method C may be used. As this ageing protocol is designed for unused oils, interpretation of test results may be difficult when ageing is performed with oil in service. However, this oxidation stability test is occasionally used to evaluate oil in new electrical equipment prior to energizing.
5.7.2 Monitoring of uninhibited oils
Oxidation of uninhibited oils is normally monitored by the formation of acidic compounds and oil soluble and insoluble sludge. An increase in DDF and reduction in IFT are also signs of oxidation of insulating oils (see 5.5, 5.6 and 5.9).
5.7.3 Monitoring of inhibited oils
Inhibited oils have a different oxidation pattern compared to uninhibited oils. At the beginning of service life, the synthetic inhibitor is consumed with little formation of oxidation products. This is referred to as the induction period. After the inhibitor is consumed, the oxidation rate is determined mainly by the base oil oxidation stability.
A decrease of IFT in inhibited oils may also be an early indication of initial formation of oxidation products.
The common and easy way to monitor the inhibitor consumption is to measure the inhibitor concentration according to IEC 60666.
The inhibitor content should be monitored at regular intervals the frequency of which will depend upon operational temperature and load levels.
5.8 Sediment and sludge
This test distinguishes between sediment and sludge.
Sediment is insoluble material present in the oil.
Sediment includes:
• insoluble oxidation or degradation products of solid or liquid insulating materials;
• solid products arising from the conditions of service of the equipment; carbon and metal particles, metallic oxides and sulfides;
• fibres and other foreign matter of diverse origins.
Sludge is a polymerized degradation product of solid and liquid insulating material. Sludge is soluble in oil up to a certain limit, depending on the oil solubility characteristics and temperature. At sludge contents above this, the sludge is precipitated, contributing as an additional component to the sediment.
The presence of sediment and/or sludge may change the electrical properties of the oil, and in addition, deposits may hinder heat-exchange, thus encouraging thermal degradation of the insulating materials.
Sediment and sludge should be measured according to the method described in Annex C.
5.9 Interfacial tension (IFT)
The interfacial tension between oil and water provides a means of detecting soluble polar contaminants and products of degradation. This characteristic changes fairly rapidly during the initial stages of ageing but levels off when deterioration is still moderate.
The rate of decrease of IFT is strongly influenced by the type of oil; uninhibited oils usually show higher IFT rates of decrease than inhibited oils.
A rapid decrease of IFT may also be an indication of compatibility problems between the oil and some transformer materials (varnishes, gaskets), or of an accidental contamination when filling with oil. However, oils with interfacial tension values at or near the lower limit value given in Table 5 should be further investigated.
With overloaded transformers, the deterioration of materials is rapid and IFT is a tool for detection of deterioration.
5.10 Particle count
Particles in insulating oil in electrical equipment may have numerous possible sources. The equipment itself may contain particles from manufacturing and the oil may contain particles from storage and handling if not properly filtered. Metal wear and the ageing of oil and solid materials may produce particles during the service life of equipment. Localized overheating over 500°C may form carbon particles. The carbon particles produced in the on-load tap-changer diverter switch may migrate by leakage into the bulk oil compartment to contaminate the oil-immersed parts of the transformer. A typical source of metallic particles is wear of bearings of the pumps.
The effect of suspended particles on the dielectric strength of insulating oil depends on the type of particles (metallic, fibres, sludge, etc.) and on their water content.
Historically, some failures on HV transformers have been associated with particle contamination. Traditional dielectric breakdown voltage tests are not sufficient to identify the problem and particle counting methods have been advised as monitoring tools (see Table B.1).
5.11 Flash point
Breakdown of the oil caused by electrical discharges or prolonged exposure to very high temperatures may produce sufficient quantities of low molecular weight hydrocarbons to cause a lowering of the flash point of the oil.
A low flash point is an indication of the presence of volatile combustible products in the oil. This may result from contamination by a solvent but, in some cases, the cause has been observed to be extensive sparking discharges.
5.12 Compatibility of insulating oils
Unused oil complying with IEC 60296 and with the same classification (class, group and LCSET as stated in IEC 60296) as that already in service should be used for topping up and/or refilling electrical equipment.
Field experience indicates that problems are not normally encountered when unused oil is added in small percentage, e.g. less than 5 %, to used oils classified as "good" (see 9.4), though larger additions to heavily aged oil may cause sludge to precipitate.
A compatibility test may be needed to determine the feasibility of mixing unused oils of different origins with oil in service. For mixing used oils, a compatibility study is strongly recommended. Reference to the oil supplier is recommended if any doubts concerning compatibility arise.
In the compatibility study, as described below, the characteristics of the mixture should not be less favourable than those of the worse individual oil.
Oils should be mixed in the same proportions as in the application, or if not known in a 50/50 ratio.
The following functional tests are recommended for each individual oil and for the mixture:
• foaming;
• oxidation stability according to IEC 61125:1992, Method C, including acidity, sludge and DDF after ageing. Test time should be according to the oil group as stated in IEC 60296;
• corrosive sulphur and/or potential corrosivity after ageing according to IEC 61125:1992 Method C.
Experience is very limited regarding the use of oil containing pour point depressants to top-up naturally low pour point oils. However, laboratory investigations suggest that no significant deterioration of low temperature behaviour is likely to occur.
Compatibility tests are particularly necessary in the case of oils containing additives. Again, reference to the oil supplier or to the equipment manufacturer is recommended.
5.13 Pour point
Pour point is a measure of the ability of the oil to flow at low temperature. There is no evidence to suggest that this property is affected by normal oil deterioration. Changes in pour point can normally be interpreted as the result of topping-up with a different oil.
5.14 Density
In cold climates, the density of oil may be important in determining its suitability for use. For example, ice crystals formed from separated water may float on oil of high density and lead to flashover on subsequent melting. However, density is not significant in comparing the quality of different samples of oil. There is no evidence that density is affected by normal oil deterioration.
Density may be useful for discriminating mineral insulating oil from other fluid types.
5.15 Viscosity
Viscosity is an important controlling factor in the dissipation of heat. Ageing and oxidation of the oil tend to increase viscosity. Viscosity is also affected by temperature. Normal ageing and oxidation of the oil will not significantly affect its viscosity. Only under extreme conditions of corona discharges or oxidation may this occur.
5.16 Polychlorinated biphenyls (PCBs)
Polychlorinated biphenyls (PCBs) are a family of synthetic chlorinated aromatic hydrocarbons, which have good thermal and electrical properties. These properties combined with excellent chemical stability made them useful in numerous commercial applications. However, their chemical stability and resistance to biodegradation has given cause for concern in terms of environmental pollution. This increasing concern over the environmental impact of PCBs has progressively restricted their use since the early 1970s and their use in new plant and equipment was banned by international agreement in 1986. Unfortunately, the use of common handling facilities has led to widespread contamination of mineral insulating oil.
The PCB content of oil in new equipment should be measured to confirm that the oil is PCB free. Thereafter, whenever there is a risk of potential contamination (oil treatment, transformer repairs, etc.) the oil should be analysed and if PCB content is found to exceed defined limits appropriate action should be taken (see 11.4).
NOTE Limits will be as defined by local regulations.
5.17 Corrosive sulphur
Table 1 identifies three methods for assessment of corrosive sulphur in oil. The IEC method is considered to be more exacting than the ASTM method and shall be passed by all oils. The ASTM method is easier to perform and may be used as an initial test but negative results may require further investigation. The DIN method is considered complementary and shall be passed in addition to either the ASTM or IEC method to be considered "non-corrosive" in Table 5.
The amount of sulphur in oil depends on oil refining processes, degree of refining and crude oil type; it is normally present as organo-sulphur, but elemental sulphur contamination can also occur. The presence of reactive compounds causing corrosion at normal operating temperatures is due to poor refining or contamination.
At relatively high temperatures, sulphur-containing oil molecules may decompose and react with metal surfaces to form metal sulphides. Such reactions may take place in switching equipment and will impact the conductivity of contacts. DIN 51353, using a silver strip at 100 °C, provides a sensitive test for such type of problem.
Some sulphur containing molecules may also cause the formation of copper sulphide (Cu2S) deposition in the paper insulation of electrical equipment. This phenomenon leads to a reduction of the electrical insulation properties and has resulted in several equipment failures in service.
Cu2S deposition occurs preferentially in paper insulated electrical equipment where corrosive sulphur compounds are present in oil, unvarnished or unprotected copper is used, operating or/and ambient temperatures are high and the amount of oxygen in oil is limited. One group of substances in oil causing this effect are disulphides, e.g. dibenzyl disulphide.
IEC 60296 provides specifications to ensure that Cu2S deposition in paper will not occur in service as a result of unused oil. The tests used for that purpose (IEC 62535 and ASTM D1275:2006, Method B) apply to oils that do not contain a metal passivator additive; Clause A.3 of IEC 60296:2012 provides a method for removal of passivators where they are present. The tests will give a positive indication if corrosive sulphur compounds are present in the oil.
Strongly aged insulating oils (e.g. with high acidity), or oils with poor oxidation stability, may give ambiguous results on the paper strip under the conditions of IEC 62535, because of heavy sludge formation. In this case SEM-EDX analysis (described in Annex B of IEC 62535:2008) may be helpful to solve ambiguous cases. False positives tests can also be avoided by carrying out the test only with insulating paper, without copper strip and comparing the paper's appearance by testing with copper.
A combination of several factors not only the potential oil corrosivity may lead to a failure in electrical equipment. In this case a risk assessment including design and operating conditions should be performed.
5.18 Dibenzyl disulphide (DBDS)
DBDS is potentially corrosive to copper surfaces at normal transformer temperatures and may form copper sulphide under certain conditions.
Among corrosive sulphur compounds DBDS appears to play a predominant role in the problem of corrosion. Identified as a major sulphur compound in several mineral insulating oils, it is present in most corrosive insulating oils produced and blended after 1988-1989 (although they passed the corrosivity tests of their time). There seem to be very few oils introduced or produced after 2006 that contain DBDS in detectable amounts.
It should be noted that there are also oils in service that are corrosive despite the absence of DBDS.
NOTE Dibenzyl disulphide is a sulphur compound used as an antioxidant additive in rubber compounds, a stabilizer for petroleum fractions and an additive for silicon oils.
5.19 Passivator
The addition of a metal passivator is the mitigation technique that has been used to the largest extent in order to minimize the risk of corrosive sulphur. In particular a toluyltriazole derivative has been used 2. Typically 100 mg/kg (0,01 % by weight) of this substance is added to inhibit the reactions of copper with corrosive sulphur.
Metal passivators, have a long history of use in mineral oil, mainly in lubricating oil but also, to a more limited extent, in insulating oil. They have been used not only to counteract corrosion, but also to improve oxidation stability and to suppress streaming electrification.
It is essential to monitor the passivator content during service.

