1 General
1.1 Scope
This International Standard describes methods for the determination of water in insulating liquids and in oil-impregnated cellulosic insulation with coulometrically generated Karl Fischer reagent.
The method in clause 2 is applicable to water concentrations above 2 mg/kg in liquids having viscosity of less than 100 mm2/s at 40 °C.
The test method in clause 3, where water is extracted by means of a nitrogen stream, is the preferred method for insulating liquids of viscosity higher than 100 mm2/s.
Clause 4 describes methods for the determination of water content in oil-impregnated paper and pressboard over the range 0,1 % to 20 % by mass.
1.2 Normative references
The following normative documents contain provisions which, through reference in this text, constitute provisions of this International Standard. At the time of publication, the editions indicated were valid. All normative documents are subject to revision, and parties to agreements based on this International Standard are encouraged to investigate the possibility of applying the most recent editions of the normative documents indicated below. Members of IEC and ISO maintain registers of currently valid International Standards.
IEC 60475:1974, Method of sampling liquid dielectrics.
IEC 60567:1992, Guide for the sampling of gases and of oil from oil-filled electrical equipment and for the analysis of free and dissolved gases.
ISO 595-1:1986, Reusable all-glass or metal-and-glass syringes for medical use - Part 1: Dimensions.
ISO 595-2:1987, Reusable all-glass or metal-and-glass syringes for medical use - Part 2: Design, performance requirements and tests.
2 Direct titration for low viscosity liquids
2.1 Field of application
This method is applicable to water concentrations above 2 mg/kg in liquids having viscosity up to 100 mm2/s at 40 °C. The precision data given in 2.10 apply only to new liquids.
NOTE 1 For liquids in service, the accuracy of the method may be affected by the presence of contaminants and degradation products.
NOTE 2 The method has been designed to be particularly suitable to hydrocarbon and ester liquids. With other liquids, particularly silicone fluids, methanol free reagents must be used.
2.2 Chemistry
The reactions occurring in a Karl Fischer titration are known to be complex, but are essentially of water with iodine, sulphur dioxide, an organic base and an alcohol in an organic solvent. The original Karl Fischer reagent used pyridine and methanol, and the reactions may be expressed as:
H2O + I2 + SO2 + 3C5H5N → 2C5H5N.HI + C5H5N.SO3 (1)
C5H5N.SO3 + CH3OH → C5H5NH.SO4.CH3 (2)
Other base-alcohol combinations are possible and may be necessary for titrations on some insulating liquids.
In coulometric Karl Fischer titration, the sample is mixed with a base/alcohol solution of iodide ion and sulphur dioxide. Iodine is generated electrolytically and reacts with water in a similar way to that shown in reactions (1) and (2). Iodine is generated in proportion to the quantity of electricity according to Faraday's law, as shown by the following reaction:
2I¯ - 2e → I2 (3)
One mole of iodine reacts with one mole of water stoichiometrically as shown in reactions (1) so that 1 mg of water is equivalent to 10,72 C (number of coulombs). Based on this principle it is possible to determine the amount of water directly from the quantity of electricity (number of coulombs) required for the electrolysis.
2.3 Apparatus
2.3.1 Principle of operation
The titration vessel has the configuration of an electrolysis cell with two compartments connected by a porous diaphragm. The anodic compartment contains the mixture of reagent-solvent and sample (anodic solution), the cathodic compartment (generator assembly) contains anhydrous reagent (cathodic solution). On both sides of the diaphragm are located the electrolysis electrodes.
NOTE Titrators without the porous diaphragm may be used.
Iodine generated by the electrolysis, as shown in reaction (3), reacts with the water in a similar way to the Karl Fischer reactions (1) and (2). The end-point of the reaction is detected by a pair of platinum electrodes immersed in the anodic solution. At the end of the titration, excess iodine depolarizes the dual platinum electrodes, giving a change in the current/voltage ratio which is used to activate the end-point indicator and to stop the current integrator.
The current integrator integrates the current consumed during the electrolysis, calculates the water equivalent according to Faraday's law, and finally displays it in micrograms of water.
2.3.2 Description of the apparatus
Commercial coulometric Karl Fischer titrators use proprietary circuitry. The following description of one suitable form of apparatus is given for illustrative purposes only.
The block diagram shown in Figure 1 illustrates the apparatus and includes the components detailed below.
2.3.2.1 Titration vessel assembly
An example of a suitable titration vessel assembly is shown in Figure 2. However, the changes in instrument technology are such that radically different designs may become available which comply with the technical requirements of this standard. The exemplified apparatus consists of:
- a flanged glass reaction vessel a) with sample injection plug b) and drain cock c) (optional);
- a polytetrafluoroethylene lid d), flanged to match the titration vessel, with three holes to receive the electrodes and drying tube;
- a generator assembly (combined electrolysis cell) e) consisting of a glass tube closed at its lower end by a diaphragm and equipped with platinum electrodes on each side of the diaphragm;
NOTE The diaphragm may consist of ion exchange membrane, fritted disc, ceramic filter or other system to prevent diffusion of both solutions, while allowing enough current for electrolysis.
- detector electrodes: dual platinum electrodes for measurement of potential or current f);
- a polytetrafluoroethylene coated stirrer bar g);
- drying tubes h) to protect the titration vessel and the generator assembly from atmospheric moisture;
- silicone rubber septa to seal the injection port. It is recommended that crosscuts should be made in the septa before use, to enable blunt, square-ended needles to be used for sample injection [see 2.4.2 d)]. Septa should be replaced as required to prevent air leakage as indicated by excessive instrument drift.
2.3.2.2 Detection circuit
DC constant voltage or a.c. constant current is supplied to the detector electrodes (dual platinum measuring electrodes) so that the end-point may be detected from the change of the polarized current or voltage.
2.3.2.3 Current regulator circuit
This circuit controls the electrolysis according to the signal from the detector circuit.
2.3.2.4 DC power supply
DC power supply for electrolysis.
2.3.2.5 End-point indicator
Indicates when the end-point has been reached.
2.3.2.6 Current integrator
Measures the quantity of electricity consumed by the electrolysis cell during the titration, then calculates and displays the quantity of water, in micrograms, corresponding to it.
NOTE Some instruments have built-in calculation facilities, and display the water concentration for a specific sample quantity.
2.3.2.7 Electromagnetic stirrer
Electromagnetic stirrer, capable of maintaining a constant speed sufficient to ensure adequate dispersion. (The content of the titration vessel will not in general be a single phase, since most insulating liquids are not completely miscible with the reagent liquids.)
2.4 Reagents and auxiliary materials
WARNING - Certain reagents may be detrimental to health and must be handled with proper care.
2.4.1 Reagents
Prepared reagents are commercially available, but care is needed that the reagent is suitable for the particular type of instrument used and the insulating liquid under test.
Interfering reactions may take place between methanol based reagents and some silicone compounds. In addition, similar reactions may take place with aldehydes, some ketones, and conjugated unsaturated organic acids, which may be present in oils as degradation products or contaminants. In these cases, reagents not based on methanol are recommended.
NOTE Some insulating liquids may require the use of additional or alternative solvents.
2.4.2 Auxiliary materials
a) Neutralizing solution, methanol containing approximately 20 mg water/cm3.
b) Desiccant, for example, anhydrous magnesium perchlorate or self-indicating silicagel.
c) Lubricant grease: Polytetrafluoroethylene based or fluorinated hydrocarbon types. Commercially supplied greases to this description have been found satisfactory.
d) Glass syringes for sample measurement and introduction. 10 cm3 and 5 cm3 syringes in accordance with ISO 595, with needles of suitable length and diameter. Needles of length 100 mm and bore 1 mm have been found satisfactory for general use. Blunt, square-ended needles are preferred, in order to minimize damage to the septa.
2.5 Preparation of the apparatus
Prepare and assemble the apparatus, install the reagents and carry out the stabilization procedure in accordance with the manufacturer's instructions.
2.6 Sampling methods
If samples taken are intended for additional tests to water content the water analysis shall be carried out first.
2.6.1 Routine sampling
For routine tests, the sampling methods described in clause 2 and 3.1 of IEC 60475 shall be used. Sample bottles shall be dried by heating in an oven for 16 h to 24 h at 115 °C +/- 5 °C.
2.6.2 Recommended sampling
For better accuracy, and particularly where the moisture content is very low, the procedures described in clause 4 of IEC 60567 shall be used. Sample bottles shall be prepared as specified in 2.7.1. The syringes and needles shall be dismantled, dried for at least 8 h at 115 °C +/- 5 °C, cooled in a desiccator with anhydrous silica gel, and kept in the desiccator until required.
NOTE The accuracy of the determination can be adversely affected by the taking of composite or average samples, which is not recommended.
2.6.3 Storage
Samples for water analysis shall at no time be exposed to direct sunlight. Between the time of collection and analysis, the samples should be kept in the dark, and the time between collection and analysis should be not greater than seven days.
2.7 Procedure
a) With samples which have been collected in bottles
Clean and dry in a well-ventilated oven at 115 °C +/- 5 °C a glass syringe and needle of a suitable size. Allow to cool in a desiccator. Fill the syringe with the insulating liquid, keeping the tip of the needle well below the surface of the liquid. Re-close the glass bottle immediately. Holding the syringe vertically with the needle uppermost, discharge all air bubbles. Discharge the contents of the syringe to waste. Refill the syringe and weigh to the nearest 0,1 g.
With samples which have been collected in a syringe.
Discharge approximately 2 cm3 to flush out the needle and weigh the syringe to the nearest 0,1 g.
The sample size is governed by the expected range of moisture content. However, the optimum sample size has been found to be 5 cm3 for most types of insulating liquids, new and used, with water contents of between 2 mg/kg and 100 mg/kg.
b) Operate the instrument controls to start the electrolysis according to the manufacturer's instructions and quickly inject a suitable quantity of sample into the titration vessel through the septum. The tip of the needle shall not be allowed to touch the surface of the reagent. Re-weigh the syringe and record the mass M, in grams, of sample injected.
Ensure that the oil is thoroughly mixed with the solvent. The speed of the stirrer shall not be changed after the instrument has self-equilibrated or during the titration.
c) Read out the quantity of water titrated, m, [expressed in micrograms (4g)] from the display when the titration is completed.
d) Carry out a duplicate determination by rinsing the syringe twice in the oil sample and refilling and weighing as in step a). Carry out steps b) and c).
e) After several determinations, a considerable quantity of insulating liquid may have accumulated. Remove the excess of liquid in accordance with the manufacturer's instructions, which should also be followed with respect to the relative levels of the anode and cathode reagents and re-stabilization of the instrument.
After several withdrawals, the titration vessel and generator electrode should be recharged with fresh solutions and the stabilization procedure repeated.
2.8 Calculation of the result
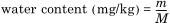
where
m is the quantity of water titrated in micrograms (μg);
M is the mass of insulating liquid in grams (g).
2.9 Report
The water content of the insulating liquid sample is the mean of the duplicate determinations, expressed to the nearest integer in milligrams per kilogram (mg/kg).
2.10 Precision
NOTE Precision data have been established for hydrocarbon based insulating liquids.
2.10.1 Repeatability
Duplicate determinations carried out by one operator should be considered suspect at the 95 % confidence level if they differ by more than 0,60 where x is the average of the duplicate determinations.
2.10.2 Reproducibility
When two laboratories carry out tests on identical test material, each shall produce duplicate results and report their mean.
The two means should be considered suspect at the 95 % confidence level if they differ by more than 1,50√x mg/kg, where x is the average of the two means.

