4 Determination of water in oil-impregnated paper and pressboard
4.1 Field of application
These methods apply to the determination of the water content in oil-impregnated cellulosic insulation materials. Three procedures are described:
- with the procedure in 4.2, water is first extracted with absolute methanol and the determination carried out on the extract;
- with the procedure in 4.3, water extraction takes place directly in the titration vessel (see note);
NOTE This procedure is restricted to cellulosic material of thickness up to approximately 1 mm because an oil-impregnated sample of higher thickness can never be completely extracted within the normal duration of a determination.
- with the procedure in 4.4, a specimen of impregnated paper is heated in an oven next to the Karl Fischer apparatus; the water vapour evolved is quantitatively transferred into the titration vessel by a stream of dry nitrogen gas.
4.2 Determination of water after previous extraction with methanol
4.2.1 Reagents and auxiliary materials
WARNING - Certain reagents may be detrimental to health and the environment and must be handled and disposed of with proper care.
In addition to the reagents and materials listed in 2.4, the following is required:
a) methanol, analytical reagent grade, water content approximately 0,05 %;
b) chlorine free suitable solvent, technical grade;
c) magnesium turnings.
4.2.2 Apparatus
a) Coulometric Karl Fischer titrator as indicated in 2.3.
b) Methanol distilling apparatus.
c) Dried methanol container, to contain the distilled methanol and which can be protected from the atmosphere by a drying tube. The container is fitted at its lower part with a draining cock and a spherical joint to adapt to the extraction tube (see Figure 5).
d) Graduated, gas-tight extraction tubes with an approximate capacity of 50 cm3.
e) Metal tweezers.
4.2.3 Preparation of the apparatus
All the glassware and metal tweezers should be washed free from previous samples, either with a suitable detergent solution or soapy water or a chlorine free solvent, rinsed thoroughly with warm water, then deionized water and finally, after draining, with methanol. The clean material should be dried in an oven at 115 °C +/- 5 °C overnight, then cooled to room temperature in a desiccator.
4.2.4 Procedure
a) Distil methanol over magnesium turnings in order to lower its water content below 200 mg/kg. Collect the distilled methanol in the appropriate container protected from the atmosphere by a drying tube filled with magnesium perchlorate. Determine the water content of the methanol and record the value.
b) Take an extraction tube containing a paper sample or put a paper sample in extraction tube.
NOTE 1 Select the mass of the sample so that the amount of water to be measured is within 1 mg to 4 mg.
NOTE 2 It may be advisable to cut up cellulosic material if it is too thick to ensure better extraction taking care to avoid any exchange of moisture with the surroundings during the operation.
c) Fit the extraction tube to the dry methanol container, open the upper cock, connect to the vacuum line for a very short time and, using an appropriate burette, admit 1 cm3 to 10 cm3 of methanol. Close the upper cock and disconnect the tube.
d) Repeat step c) with another tube that does not contain any paper. This will be used for the blank test.
e) Shake the sample and blank tubes for 2 h.
f) Operate the Karl Fisher titrator as per procedures specified in 2.5.
g) Fit the sample tube to the titration vessel, transfer the methanol to the solvent and titrate to the end-point. Read the mass of water titrated (m2) from the display on the titrator.
h) Repeat step g) with the blank tube and record the display reading (m1).
i) Remove the paper from the titration tube. Degrease the paper with chlorine free solvent and dry at 110 °C +/- 5 °C for at least 2 h. Let the paper sample cool in a desiccator and then weigh. Record the mass M, in grams, of the paper.
j) Carry out a duplicate determination.
4.2.5 Calculation of the results
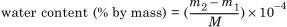
where
m1 is the mass of water measured for blank titration, in micrograms (μg);
m2 is the mass of water measured in the sample titration, in micrograms (μg);
M is the mass of the paper sample, in grams (g).
4.2.6 Report
The water content of the insulation is expressed as the average of duplicate measurements to the nearest 0,01 %. The water content of the methanol used shall be quoted.
4.3 Determination by direct titration
4.3.1 Reagents and auxiliary materials
In addition to the reagents and materials listed in 2.4, a suitable chlorine-free solvent is required for degreasing the sample material.
4.3.2 Apparatus
a) Coulometric Karl Fischer titrator as described in 2.3.
b) Metal tweezers.
4.3.3 Procedure
a) Operate the Karl Fischer titrator as per procedures specified in 2.5.
b) Introduce, as quickly as possible, the paper sample by means of dried metal tweezers into the titration vessel.
NOTE See notes 1 and 2 item b) of 4.2.4.
c) Turn off the titration switch on the main unit, or otherwise inhibit titration, for a time period ranging from 15 min to 25 min (the exact time shall be determined by the operator) to allow transfer of moisture from the paper to the solvent. At the end of the extraction time, turn on the titration and titrate to the end-point.
d) Read the quantity of water titrated (m2) from the Karl Fischer apparatus.
e) Carry out a blank test of the same duration in order to evaluate the amount of moisture that enters the system during the test. Record the mass (m1) titrated.
In some designs of titrator, the drift is continuously monitored and automatically subtracted from the water reading. If so the blank test may not be needed though care should be used to ensure stable operating throughout the titration.
NOTE It is important to limit to a minimum the duration of the determination, which can be fixed by the operator on the basis of experience and the type of sample examined. If necessary, the optimum period can be determined by repeatedly carrying out the procedure using increasing extraction times until a constant result is obtained. To obtain reproducible results over a series of tests, the same extraction period should be used throughout the series.
f) Remove the paper from the titration tube. Degrease the paper with the solvent and dry at 110 °C for at least 2 h. Let the paper sample cool in a desiccator and then weigh it. Record the mass M, in grams, of the paper.
g) Carry out a duplicate determination.
4.3.4 Calculation of results
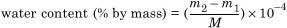
where
m1 is the mass of water measured for blank titration in micrograms (μg);
m2 is the mass of water measured in the sample titration in micrograms (μg);
M is the mass of the paper sample in grams (g).
4.3.5 Report
The water content of the insulation is expressed as the average of duplicate measurements to the nearest 0,01 %. The time of extraction shall be quoted.
4.4 Evaporative stripping method
4.4.1 Apparatus
The block diagram of the apparatus is illustrated in Figure 3 with components as listed in 3.3.
The boat type of evaporator using a gas-solid extraction is also suitable.
4.4.2 Procedure
a) Operate the Karl Fischer apparatus as per procedures specified in 2.7.
b) Heat the evaporator to a suitable temperature (130 °C for low viscosity oil impregnated paper or pressboard, 140 °C for paper or pressboard impregnated with viscous oils or compounds).
c) Adjust the carrier gas flow so as to ensure a steady flow rate of 50 cm3/min to 100 cm3/min. Insert the tip of the carrier gas outlet tube into the titration vessel and allow the system to stabilize as indicated by a low and stable reading of background current (drift).
d) Quickly introduce the paper sample taken from a storage flask into the evaporator vessel. For papers with very low moisture content a paper sample of about 0,5 g is adequate.
e) Turn off titration switch on main unit, or otherwise inhibit titration, for a 20 min period to allow moisture released in the evaporator to accumulate in the titration vessel. At the end of the extraction time, switch on titration and allow to titrate to the end-point. Read the quantity of water titrated (m2) from the Karl Fischer apparatus.
NOTE Extraction time will depend on sample characteristics as well as system parameters. Although a 20 min period was found adequate in most cases, the operator will have to determine optimum operating conditions.
f) Carry out a blank test of the same duration in order to evaluate the amount of moisture that enters the system during the test. Record the mass titrated (m1).
In some designs of titrator the drift is continuously monitored and automatically subtracted from the water reading. If so the blank test may not be needed though care should be taken to ensure stable operating throughout the titration.
g) Remove the paper from the titration tube. Degrease the paper with the solvent and dry at 110 °C +/- 5 °C for at least 2 h. Let the paper sample cool in a desiccator and then weigh it. Record the mass M, in grams, of the paper.
h) Carry out a duplicate determination.
4.4.3 Calculation of results
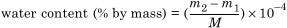
where
m1 is the mass of water measured for blank titration in micrograms (μg);
m2 is the mass of water measured in the sample titration in micrograms (μg);
M is the mass of the paper sample in grams (g).
4.4.4 Report
The water content of the insulation is expressed as the average of duplicate measurements to the nearest 0,01 %. The time of extraction shall be quoted.

