ABSTRACT: Antioxidant chemistry is playing an important role in meeting the longer-life and higher temperature performance criteria of modern generation turbine lubricants. Oxidative life assessment is therefore an important parameter as part of today's turbine oil diagnostic services, where methods such as RPVOT, FTIR and RULER are being applied.
With this paper, we will discuss how RPVOT (per ASTM D-2272) testing of turbine lubricants with single antioxidant systems have much better reproducibility and repeatability than comparable RPVOT testing of oils with complex, synergistic mixtures of antioxidants. Both new and used turbine oils obtained from gas and steam turbines were used to assess the effects of antioxidant chemistry on the RPVOT results.
In the first part of this research program, we present the analytical results for the residues remaining at the completion of the RPVOT tests of single component antioxidant oils. The RULER, varnish potential index (VPI), FTIR, AN and viscosity analyses of the residues are very similar indicating that the residues contain no remaining antioxidants and have elevated levels of VPI, AN, viscosity and FTIR oxidation, i.e., all of the RPVOT residues of the single antioxidant oils are highly oxidized when the test is ended based on oxygen uptake.
In the second part, we present the analytical results for the RPVOT residues of complex mixture antioxidant oils. In contrast to the single antioxidant oils, the analytical tests of the complex antioxidant RPVOT residues indicate that the concentration of antioxidant and the level of oxidation varies with antioxidant formulation when the test is ended based on oxygen uptake.
The results presented in this paper indicate that the effects of antioxidant chemistry on the residue content of RPVOT tests help explain the poor reproducibility of the RPVOT tests for turbine oils containing different types of antioxidant systems. Consequently, RULER analyses to characterize the antioxidant systems of the turbine oils before and after RPVOT testing would be very valuable in interpreting the RPVOT results and in improving the reproducibility of the RPVOT technique for making oxidative life assessments of modern generation turbine lubricants. VPI assessments of the turbine oils would further improve the oil diagnostic services of turbine lubricants by providing insight into the capability of the lubricants to solubilize the oxidation products of the antioxidants and the highly-refined base-oil.
KEYWORDS: antioxidants, remaining useful life, voltammetry, RPVOT, varnish index potential, oxidation, phenols, aromatic amines, steam turbines, gas turbines
Introduction
There have been countless instances of problematic turbine engine failures that have yielded used oil samples with a darker than normal appearance and a foul odor, yet conventional used oil testing of these samples has shown quite normal test results. Even on normally operating turbine engines, the routine predictive analysis of the lubricant for Remaining Useful Life has been considered to be less of a science, and closer to an art form, for many decades. The most popular test for measuring oxidation stability, Rotating Pressurized Vessel Oxidation Test (RPVOT), has been widely used and touted as an industry standard, while at the same time, the test results are often ignored, especially when the results come into conflict with other test data or other operating criteria. Typical examples of this phenomenon, are when the RPVOT of the new oil, is lower in value than the RPVOT of the in-service oil, or when the in-service oil RPVOT results are far out of range with the new oil data, yet the oil has only been in use for a short period time and no other abnormal parameter can be found with the oil. Some real-life RPVOT data are given in this paper in the section titled RPVOT Reproducibility Issues. It is the authors experience that true, blind, inter-laboratory round-robin testing for RPVOT, can not achieve the Reproducibility values as specified by ASTM D-2272 method.
The RPVOT test is designed to be a performance test for measuring the remaining test life of in-service oils. It takes into account the natural anti-oxidative properties of the base-oil as well as the oxidation inhibiting capabilities of the Anti-oxidant additive for the base-oil. The RPVOT does this by stressing the oil in a pressure vessel with oil, water, copper catalyst, heat and pure oxygen. The test is considered to be complete (formulated and natural antioxidants depleted), when the oxygen pressure drops (base-oil undergoing rapid oxidation) by a specified amount below the maximum pressure developed. Therefore, once the test is completed, the antioxidants should be totally depleted and the base-oil undergoing accelerated oxidation resulting in increased acid number (AN) and Viscosity values with respect to the new oil. Since inter-laboratory testing found poor Reproducibility values for RPVOT, the authors took a closer look at the residual oils from different RPVOT tests and found that the AN and Viscosity values of numerous residual oils were not increased with respect to the new oil. Consequently, it was decided to investigate further into this phenomenon by studying the oils as brand name groups, and to widen the scope of testing of the residual oils, to include FTIR spectroscopy (base-oil oxidation),Voltammetry (antioxidant depletion) and colorimetric stain (Varnish Potential Index) analyses.
It was discovered that 6 different types of oils using mono-type Anti-Oxidant additive packages were fully oxidized during the RPVOT test, as measured by the AN, Kinematic Viscosity, FTIR spectroscopy, Voltammetry and Varnish Potential Index. These oils are called Sensitive to the RPVOT test. In contrast to the mono-type additive packages, 3 different types of oils using complex (Synergistic) Anti-Oxidant additive packages were found to not fully oxidize during the RPVOT test, as measured by the same criteria. These oils are called non-Sensitive to the RPVOT test.
This paper demonstrates that while the exact Anti-Oxidant chemistry of branded turbine oils remains as trade secrets of the oil refiners, there should be an assessment made, by the oil refiner, of whether or not, their brand of oil is sensitive or non-sensitive to RPVOT testing. This assessment should then be made part of the product Specification sheet. This will help clear up much confusion in the minds of many turbine engine owners and operators. It is also recommended to the ATSM committee, that further study may be required to answer the question; can the RPVOT test method be modified to allow testing of non-Sensitive oils?
1. The important Role of Antioxidants in Modern Lubricants
When modern turbine lubricants are developed, the lubricant manufacturing company has an important role of developing a lubricant which shall correspond to the particular turbine's performance requirements. Basically that means that an important role is dedicated to the development and selection of the base oil in combination with the selection of the additives. Why? In order to meet the basic lubricant requirements, and to protect against oxidation, corrosion, temperature extremes, wear and deposits, oil additives are blended to the base oil. These are usually known as anti-wear, extreme pressure, antioxidant, dispersant, anti-foam, and VI-improver additives, all of which, have their own specific roles. On the other side, as a direct result from increasing operating economy parameters, OEMs'have driven the lubricant requirements toward more demanding specifications due to:
Increased load factors on new & existing types of equipment
Extended operational service/maintenance periods
Extended drain intervals
Decreased lube reservoir volume (cost/weight/engineering reasons)
The demanding specifications challenge new generation of turbine lubricants to possess greatly improved capabilities to inhibit oxidation and explains why in the last decennia a wide variety of new antioxidant technologies have been emerging to respond to this market need. Until the effects of the new antioxidant technologies on long-term lubricant performance and traditional condition monitoring techniques have been fully established, there will be a need of measuring and trending their concentration in order to make proper Remaining Useful Life evaluations of in-service lubricants. For this paper, Remaining Useful Life or Remaining Oxidation Stability is the length of time the lubricant can be used before the antioxidant(s) become depleted allowing rapid base-oil oxidation signified by increased TAN and viscosity values, lubricant darkening, deposit formation, etc. Using this definition, new oil has 100% remaining useful life and caused oil with elevated TAN values has 0% remaining useful life.
1.1 Which Type/classes of Antioxidants are used?
Before explaining in more detail the different classes of antioxidants and their principle of working, it is vital to understand that in many modern lubricants a mixture of antioxidants is applied. Therefore it will be important to understand the total oxidative health of the lubricant, and not just one type of antioxidant.
What is oxidation? Oxidation is the chemical reaction of a lubricant at elevated temperatures between the dissolved atmospheric oxygen and the base-oil (figure 1a). During the oxidation, as hydrocarbon molecules will break down, reaction products will be formed, better known as radicals (very reactive chemical compounds). Subsequent reactions of these radicals lead to the formation of peroxides, and must therefore be quenched by the antioxidants to preserve the lubricant integrity, or its Remaining Useful Life, RUL. Most of us relate the oxidation (or aging) of an oil with the formation of acids (figure 1b), resins or other nasty chemical compounds, which have to be seen as final reaction compounds, or when it's too late to react. We will come back on the importance of RUL, in the following paragraph.
The role of antioxidants is to protect the base oil by either scavenging these radicals or decomposing hydroperoxides into stable products. This is very similar to the human body where vitamins (fresh vegetables, fruit, and wheat) are excellent antioxidants to neutralize these reactive compounds. Continuously refreshment of your body with fresh vitamins can be compared with the top-off technique applied on lubricants. At the same time literature refers to negative effects of over-additization, just like for a human body.:
Primary antioxidants - remove the radicals (known as radical scavengers) that initiate the chain reaction that results in accelerated lubricant oxidation. Hindered aromatic amines and phenols are characteristic types of primary antioxidants, widely used in industrial lubricants. Therefore these types of ashless antioxidants go straight to the root of the problem and prevent deposits from forming in the first place.
Secondary antioxidants - react with peroxides (known as hydroperoxide decomposers) and to form non-reactive products that do not participate in further oxidation of the lubricant. Zinc dithiophosphates, or better known under the name of ZnDtP or ZDDP, phosphites and phosphonites belongs to this class of antioxidants.
Mixed antioxidant systems. When two or more antioxidants are added to oil, an antioxidant effect is frequently observed in excess of either additive introduced individually. Antioxidants are often used in synergistic mixtures in modern lubricant formulations, to achieve an extended useful life, where one of the antioxidants sacrifices itself in preservation and regeneration of the other. A realistic example is the synergy between amines and phenols whereby the hindered phenols give excellent protection at low-temperature regimes (and deplete first), while the amine antioxidants are more effective in extending lubricant life at higher temperature ranges.
In the above figure 1 a, the oxidation accelerators (heat, water, air and metals) play an important role, as they can act separately or in combination, but are very much application dependent (will also be discussed in the practical case studies). Lubricating oils and greases oxidize in much the same manner, and the oxidation process steps are very much temperature dependent. The antioxidant (inhibitor) reaction mechanism scavenges the free radicals to stop the formation of oxidation products (carbonyl compounds in figure 1b). The actual mechanism will depend upon the type of antioxidants applied and selected.
With the continuous increase of power plants efficiency, as well size of power plants, thermal loads of turbine oils have been rising over the last 10 years. This has resulted in a large change in new lubricant specifications for gas and steam turbines lubricants. The gas turbine lubricants inherently work at higher operating temperatures, and are faced with higher oxidative stresses. The steam turbine oils do not follow the same temperature evolution as for the gas turbines, but have also seen an increase of operating temperature over the years, requiring a higher oxidative stability (e.g. combined cycle power plants). Because turbine oils are increasingly required to have longer life at higher operating temperatures, turbine oils with amine additives as a mixture with phenolic antioxidants are coming into use for steam turbine, gas turbines, as well for combined cycle turbines.
Also with the increases of operating temperatures and equipment availability, turbine lubricating equipment manufacturing companies have started to include new proactive parameters in their maintenance specifications. Not only will these parameters result in a better balance between equipment and oil health monitoring, but also increase the availability of the equipment. This explains why oxidative health monitoring will be of high economic value for in-service oils as well for incoming oil batches.
1.2 The value of monitoring antioxidants - to know the RUL or not to know the RUL, that's the question?
The answer to this question is found in the basic characteristic of modern Maintenance Techniques, which require Root Cause Failure analysis. In order to help extend fault free machine operating-life, the trending of oxidative health, or antioxidants concentration, will be required to look at the root causes of lubricant failures. Typically oxidative health is monitored by AN tests which have a very low proactive value in CBM programs. Viscosity increase, which is a direct result from the polymerization (chain formation) between hydrocarbon (base-oil) chains and enhanced by the oxidation products is a second indicator or signal of heavy lubricant degradation exists.
The main drawbacks of techniques like AN, Viscosity and FTIR-Ox is their inability to predict the operating time from when the fluid was sampled and tested, until a fluid change will become necessary due to additive depletion. These tests have very low-slope rates-of-change, and tend to show completely normal results until the very latter stages of the oils' life. When critical oxidative depletion finally starts to take place in the oil, these tests take on a rapid rate-of-change or slope, but they are usually not noticed, because the fluid is not sampled frequently enough to detect the initial rapid rate of change. In addition to that, an AN increase of just 0.1 AN, from 0.1 AN to 0.2 AN, can be significant, yet the AN test has accuracy problems in this range.
By monitoring the antioxidants, lubricant operators will detect additive failure in advance of oxidation, acid formation, thickening and varnishing so as to avoid secondary component failure caused by accelerated wear, corrosion, filter plugging and bearing lubricant starvation. And herein lays the major benefit by monitoring the antioxidant concentration or the Remaining Useful Life (RUL), as users will be able to look forward, rather than look backward by being reactive on changes of parameters like viscosity, AN or oxidation by FTIR (FTIR-Ox).
This is why, in contrast to conventional fluid degradation techniques, other techniques are required which can routinely monitor the antioxidant concentration in a predictive mode. These tests need to be able to trend results well, with easy to recognize data slopes that give sufficient early warning, warning times that are well within the acceptable sample frequency interval.
For each type of turbine, antioxidant depletion rate will reflect the turbine's characteristic operating conditions enabling operators to look at the root causes for possible abnormal conditions. Experiences have shown that with 10-20% remaining antioxidant concentration, especially with higher temperature applications, large changes in the basestock's physical properties occur, i.e. the useful life of the oil ends. If a lubricant is than used past its end of useful life, excessive basestock degradation can occur, resulting in component wear and eventually equipment/engine malfunctionbetter known as oxidative accelerators can be divided in 3 categories:
Temperature stress - elevated temperature is an important accelerator to oil oxidation. This can be due to local hot spots (local bearing effects, dieseling), or overall high operating temperature. The impact of high temperature on the rate of oil oxidation (rule of Arrhenius - for operating temperatures higher than 100°C/170°F each increase by 10°C/17°F, will double the rate of oxidation or half the oxidative life of the lubricant)
Solid contamination (through wear debris or dirt ingestion) - accelerate the oxidation as being catalysts and decompose hydroperoxides.
Water contamination acting as oxidative accelerators - Moisture/Water contamination (due to ingestion, condensation, and fresh lubricant top-up) and hence the importance of combining antioxidant trend analysis, with predictive maintenance techniques like wear/contamination and water.
These oxidative accelerators will enhance the fluid degradation and will increase the degradation when they work in combination, like water and metals. see figure 1b above:
(1) With equipment conditions changing continuously lubricant suppliers are changing the lubricant formulations to meet these demands.
(2) To detect faulty storage conditions of the fluids resulting in fast (auto) depletion of antioxidants
(3) To assist lubricant operators during normal oil top-off operation and avoid mixing of lubricants.
2. Existing Techniques to monitor oxidative health of turbine lubricants For turbine oil oxidation assessment, as part of oil condition monitoring practices, different techniques are applied, from which 2 will be evaluated in this paper: RPVOT (Rotating Pressure Vessel Oxidation test as per ASTM D-2272), and Voltammetric techniques (RULER as per ASTM D-6971)
2.1 RPVOT test method (per ASTM D-2272)
The most common test for turbine oxidative life measurement is the rotating pressure vessel oxidation test (RPVOT) as per ASTM D-2272. This test involves placing a sample of oil into a rotating pressure vessel along with a concentration of water and a copper coil. The vessel will be pressurized at a pressure of 90 psi with pure oxygen, and placed into a heating bath set at 150°C on a device that rotates at 100 rpm. As the temperature of the pressure vessel and its content increases, the pressure increases, until it stabilizes, and whereafter the test will start. During the RPVOT test, the oil's ability to resist oxidation degrades as a result of stress-induced antioxidant depletion, to the point where the base oil start to react with the oxygen as the oil molecules begin to oxidize. At that point the pressure drop in the pressure vessel starts to accelerate and when the pressure drop reachs a value of 25 psi, this will be known as the end-point of the RPVOT test. The time in minutes is reported as the oil's RPVOT value, and should be directly linear to the depletion of the antioxidant additive package which is degrading during operation. Consequently the number of minutes required to reach the RPVOT end point decreases as an oil begins to age in-service and indication a loss of the RUL.
Initially the RPVOT test was used on turbine oil formulation using phenolic mixtures resulting in RPVOT test times between 300 and 600 minutes. Over the last decade, turbine generator sets have been characterized by a significant increase in operating temperature, as well life time, which resulted into the introduction of mixtures of aromatic amines and phenols. With these synergetic mixtures, the RPVOT values have significantly increased to values varying between 800 and 3000 minutes.
2.1.1 RPVOT Reproducibility Issues
To test the Precision and Bias Statement of ASTM D2272 (the RPVOT test), it was decided to conduct blind and non-blind testing of new and used turbine oils in four different laboratories in four different parts of the world. Samples of a new gas turbine oil containing a phenolic/aminic mixture antioxidant package with a posted RPVOT value of 1400 and two (2) similar formulation used gas turbine oils were sent to four (4) well established petroleum testing laboratories in different regions. Only one of the laboratories was told the brand and type of oil, and which sample was new oil and which samples were used oils. The results of this testing are given in Table 1 below.
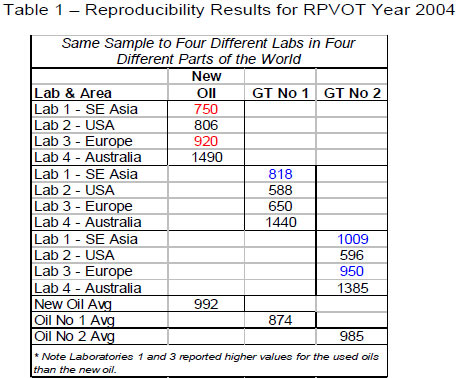
It is interesting to note that the only laboratory that was able to successfully report the results in the correct sequence of new vs. used oils with the expected RPVOT ranges was the one laboratory that was told the oil type and which samples were new and used. A conclusion from this data could be that in true blind situations, the RPVOT test is not capable of detecting new oil from used, on that particular type of oil.
2.2 Voltammetric techniques (RULER, per ASTM D-6810, D-6971) for direct detection of antioxidant chemistry
The working principle of the RULER™ method is based on voltammetric analysis (6,7,8,12) in which the oil sample is mixed with an electrolyte and a solvent, and placed in an electrolytic cell to detect the electrochemical (antioxidant activity). The oil samples (max. 400µl) are diluted in an acetone/electrolyte mixture (RULER™) Green test solutions) enhancing extraction of the antioxidants (AO[s]) into the solvent phase. When performing a voltammetric analysis, the potential across the electrodes varies linearly with time, and the resulting current is recorded as a function of the potential. With increased voltage to the sample in the cell, the various additive species under investigation in the oil oxidize electrochemically. A typical current-potential curve produced during the practice of the voltammetric test is illustrated in Figure 2. Initially the applied potential produces an electrochemical reaction with a rate so slow that virtually no current flows through the cell. As the voltage is increased (Figure 2), the electro-active species (such as antioxidants) begin to oxidize at the microelectrode surface, producing an anodic rise in the current. As the potential is increased (from 0 to 1.7 V at a rate of 0.1 V/second), the decreases in the electro-active species concentration at the electrode surface and the exponential increase of the oxidation rate lead to a maximum in the current-potential curve (Figure 2); this is the oxidation wave. The data recorded during this oxidation reaction can then be used to predict the remaining useful life of the lubricant, or used to evaluate the remaining antioxidant additives of the used samples. The peak of a zinc dialkyl dithio phosphate (ZDDP) additive is followed by an amine (PANA), and then by a hindered phenol (BHT) (see figure below).
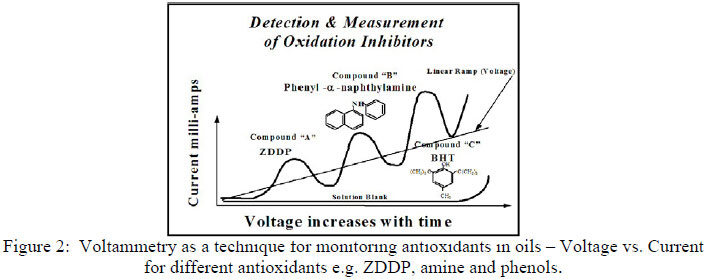
Figure 2: Voltammetry as a technique for monitoring antioxidants in oils - Voltage vs. Current for different antioxidants e.g. ZDDP, amine and phenols.
2.2.1 Voltammetric test procedures
To enhance the extraction of antioxidants out of the oil phase, the following RULER test procedures have to be applied:
(1) Dispense 400µl of the oil sample inside the vial, containing the electrolytic solution
(2) Shake vial for 10 seconds.
(3) Let solution settle for about 2 minutes until clear liquid is evident on top
(4) Perform RULER test. The fresh lubricant is used as the 100% standard and the measurements of the used lubricant samples were expressed as percentage remaining additives (see figures 6 and 10).
While ASTM D-6810 specifically covers the measurement of phenolic inhibitors in turbine oils, ASTM has approved a second standard, ASTM D-6971, to measure the concentration of phenolic and aromatic amine antioxidants in non-zinc containing turbine oils.
Voltammetric test practices are also part of ASTM practices for steam and gas turbine lubricant monitoring (ASTM D4378-03 and D6224-02)
The RULER instrument is also perfectly capable of measuring the oxidation stability provided by ZDDP and similar antioxidant/antiwear additives.
3. Experimental
With purpose of developing a balanced formulation between base oil and additives (in this case, more specifically the antioxidants) we can make an assumption that a turbine oil will truly be oxidized up to, or beyond, the critical point when antioxidants have been consumed, and the base oil will be undergoing secondary degradation (due to the lack of antioxidants to neutralize the C-radicals). It is well accepted that beyond this point, the AN and Viscosity oil parameters will show a significant increase over their new oil values.
The experimental work from this paper focuses on the reproducibility issues with RPVOT by taking a closer look at the residual oil that comes out of the RPVOT apparatus, after the test has been completed. The rationale behind doing this, is that one could reasonably expect all oils that come out of the RPVOT test apparatus will be depleted of antioxidants and there will be a sharp increase in AN and Kinematic Viscosity. When it was noted that this was not particularly the case, it was decided to investigate further into this phenomenon by studying the oils as brand name groups, and to widen the scope of testing of the residue, to include FTIR spectroscopy, Voltammetry and Varnish Potential Index (colorimetric stain analysis).
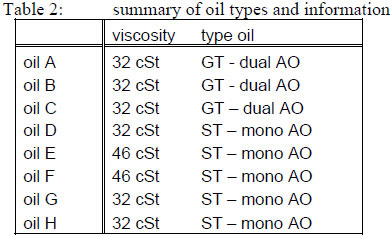
Turbine oils - table 2 below describes the following 8 oil types which were evaluated in the program, consisting of 3 types of gas turbine oil (GT) (with dual antioxidant package), and 5 steam turbine oils (ST) with mono formulation of antioxidants. All of the oils belong to the Group II or Group III baseoils.
Voltammetry - in this research program test results were measured by using a commercially available voltammograph (RULER technology), as per ASTM procedures D-6971. Neutral electrolytic test solutions were used to monitor both aromatic amines as well phenolic antioxidants, and the FLUITEC R-DMS data acquisition software was used for automatic RUL% calculation per additive.
RPVOT test instrument - RPVOT tests were performed by using a Koehler 4-vessel RPVOT Instrument per ASTM D-2272. The pressure vessels were equipped with newer-style digital transducers. The data inflection points were acquired using the Oxidata V 7.2 software. After completion of the RPVOT test, the residue for the pressure vessel was collected for further analysis.
VPI - A proprietary colorimetric stain technique developed by Focus Laboratories Ltd. The scale is 0 to 100 VPI, with 0 being new clean oil, and 100 being a critical point of varnish residue. The test is a trending tool for the early signs of varnish precursors.
FTIR - Analytical spectra were taken using a Bio-Rad FTIR instrument. The sample pathlength was 0.1mm. The oxidation area of measurement was 1800-1670 cm-1 with two baseline correction points; left of 2200 to 1800 cm-1 and the right as 650-550 cm-1. The nitration area was 1650-1600 cm-1, with the same baselines as oxidation. The analysis was in accordance with JOAP and the instrument manufacturers' procedures.
Viscosity - Kinematic viscosities at 40°C were measured using a Cannon CT 500 Viscosity apparatus, using Zeitfuchs crossarm viscometers. The method conforms to ASTM D-445 method.
Acid Number (AN) - Acid numbers were performed using ASTM D974, manual colorimetric titrations. The samples were dissolved in a solution of toluene/isopropyl alcohol/water and titrated to their end-points using a standard alcoholic base solution and p-naphtholbenzein indicator solution.
4. Results and Discussions
4.1 RPVOT comparison from mono-antioxidant formulated type of turbine oils The first part of the research paper focused on the mono-type of antioxidant inhibited lubricants (voltammetric analysis highlighted that the antioxidants, were mostly phenolic type of antioxidants). To assess the feasibility of the RPVOT technique for implementation in a used oil program, the RPVOT results were compared to the voltammetric results (RUL%) per type of antioxidants, AN, FTIR (oxidation), VPI, and viscosity. For this comparison a total of 26 used oil samples were obtained from different operational power plants and different oil suppliers.
Figures 3, 4 and 5 below present the RPVOT test results (% RPVOT =used oil RPVOT result (min)/ reference oil result (min) x100%) versus the analytical test data for the remaining antioxidants (RUL%), viscosity, AN, Oxidation by FTIR and VPI, for D,G and H oils. As all the data were similar in between the oil types D,E, F, G and H we only present a selection of data below (figures 3, 4, and 5).
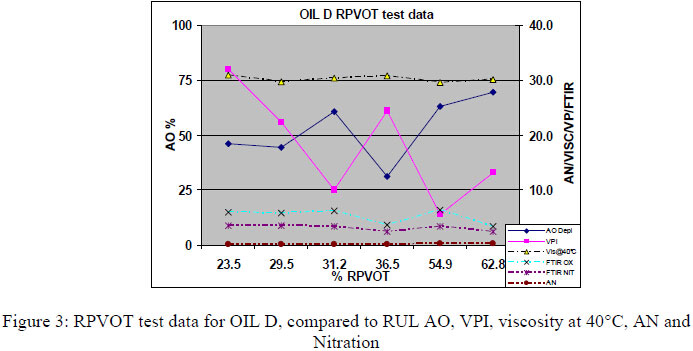
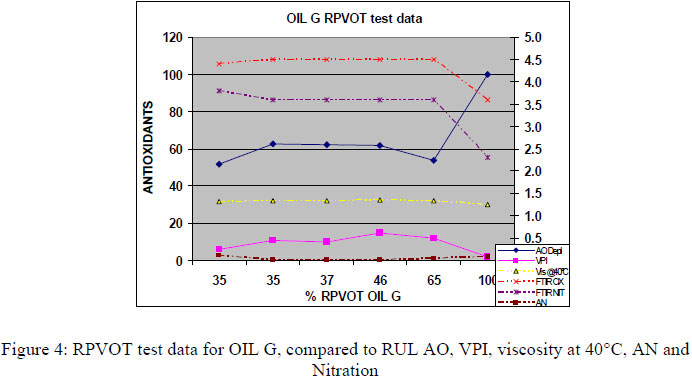
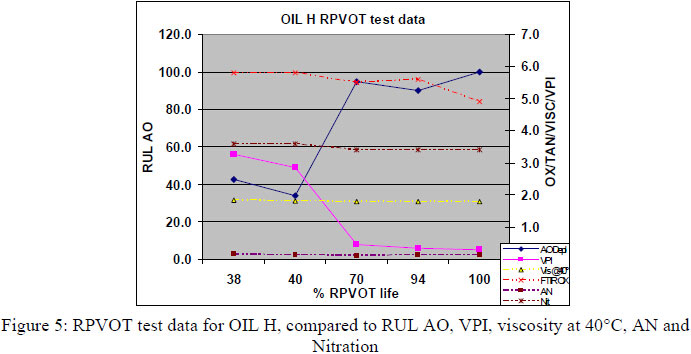
From the graphs (Figure 3, 4, and 5) above we can see a good correlation between RPVOT and remaining antioxidant activity. Secondly the VPI test data shows the first increase of varnish potential with lower RPVOT and Antioxidant concentration. Viscosity, oxidation by FTIR and Acid number test data did not increase very much, as the turbine base oil was still having enough oxidative protection by the remaining concentration of antioxidants. Consequently, to quantify and measure the antioxidant depletion after the RPVOT test, we have analyzed the residue oil samples (Residue means that the sample is the oil that was returned from the RPVOT apparatus, after the RPVOT was completed) enabling us to perform following tests:
(1) RULER test to quantify the remaining concentration of antioxidants by comparing the voltammetric response for the fresh oil vs. the used oil
(2) Oxidative degradation by analyzing the VPI, Viscosity at 40°C, FTIR oxidation and nitration, and Acid number.
On the residue oil samples, the Ruler voltammograph (figure 6) shows no remaining antioxidants concentration and is also confirmed by the physical data: high viscosity increase, high acid number increase and high FTIR oxidation etc. This confirms for these type of turbine oils that we monitored a total depletion of antioxidants, after a RPVOT test was performed.
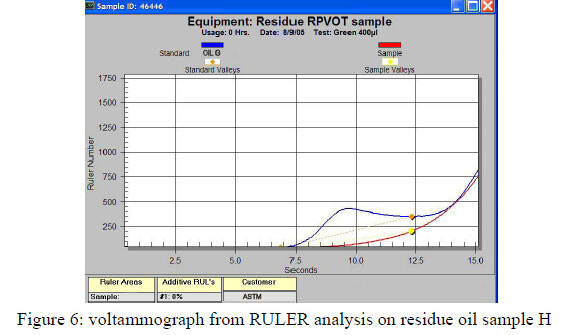
As the results for the oils D, F, E, G and H are very similar we only present below a selection o the residue oil analysis data - Tables 3, 4 and 5 below presents the data from residue oil samples from Oils H, D and G and with their different monitored analytical parameters.
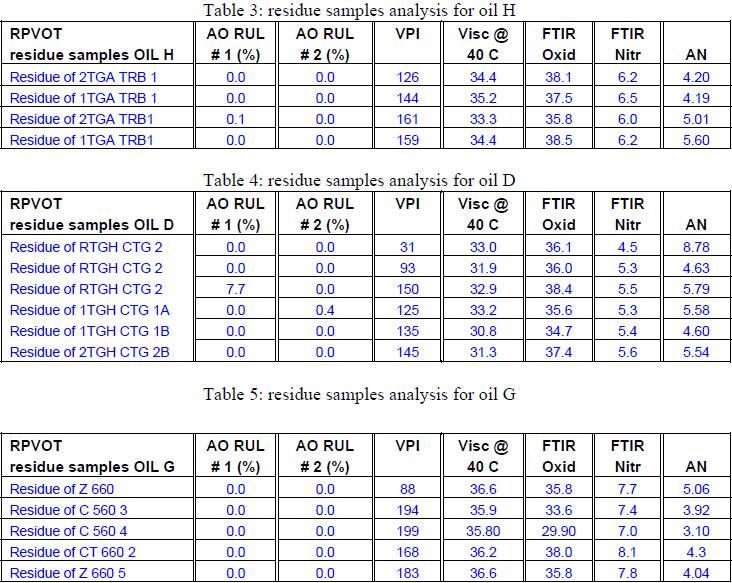
For all the samples being tested the residue oil samples showed a total antioxidant depletion as well a significant increase of VPI, Viscosity, Oxidation by FTIR, and Acid number. This is a logical consequence of the base oil degradation, and lack of antioxidants. In the following paragraph we will start comparing data for multiple antioxidant formulations.
4.2 RPVOT comparison from synergetic (multiple) antioxidant formulated type of turbine oils. For the second part of this research paper a total amount of 32 oil samples, sampled from different Thai power plants were also first analyzed through the RPVOT test instrument. 3 different types of turbine oils, blended with multiple antioxidants, were included in this research program. As in paragraph 7.1, we have performed RPVOT testing on their 32 used and new oil samples, including VPI, viscosity, FTIR Oxidation and nitration, as well Acid number. The graph below correlates the RPVOT test results (% RPVOT ), vs. the remaining antioxidants (RUL%), as well viscosity, TAN, Oxidation by FTIR and VPI, for 3 different types of oil (oils A, B and C)
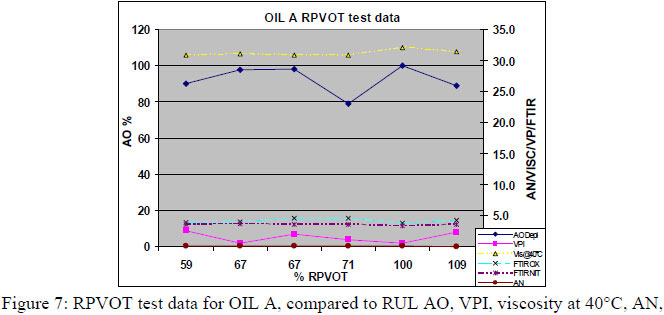
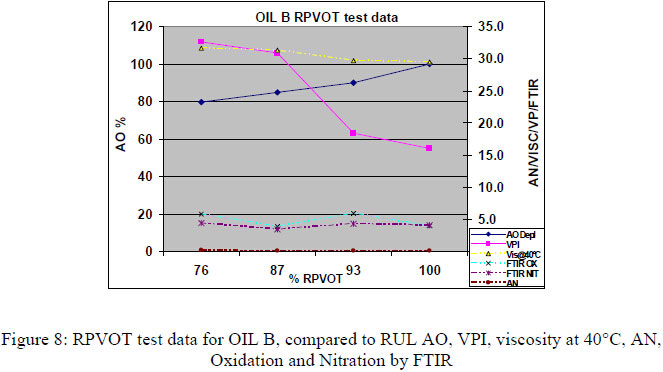
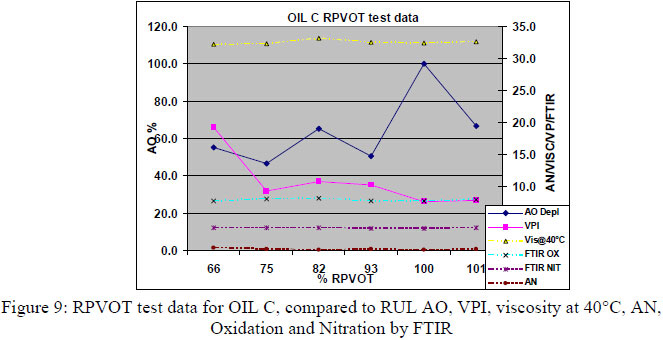
To quantify and measure the antioxidant depletion during the RPVOT test, we have taken from all the RPVOT tests that we performed, the residue oil samples, enabling us to perform following tests:
(1) Voltammetric (RULER) analysis to quantify the remaining antioxidants
(2) Oxidative degradation by analyzing the VPI, Viscosity at 40°C, FTIR oxidation and nitration, and Acid number.
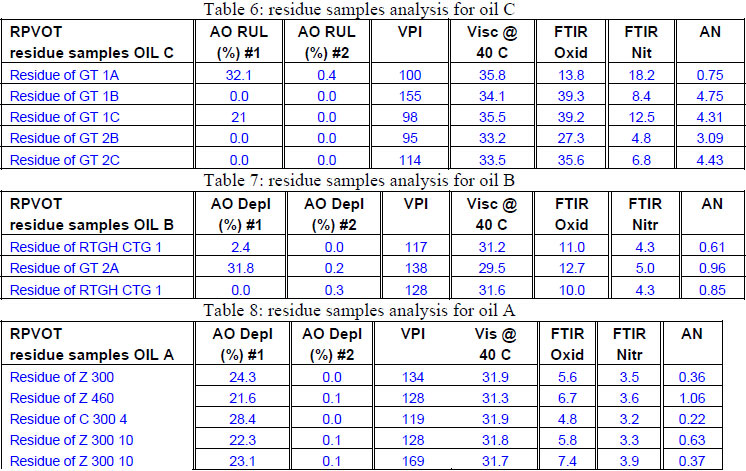
For the majority of these oils we monitored a partially depletion of antioxidants, as well a difference in increase for the oxidation detected by FTIR, and oxidation acids by AN. A typical example can be seen in Figure 10 below which shows a voltammograph from a RULER analysis, for oil type A residue after performing a 3000 minutes RPVOT test. The blue (upper) curve represents the fresh oil with antioxidant # 1 consisting of an aromatic amine and the antioxidant #2 a phenolic type of antioxidant. The red (lower) curve shows the RULER response for the remaining activity of antioxidants, where a 28.4% remaining activity of aromatic amines is analyzed, and the phenolic antioxidants are totally depleted. This residue oil sample had also the lowest AN increase, as well oxidation increase by FTIR, but showed the first signs of Varnish Potential Increase.
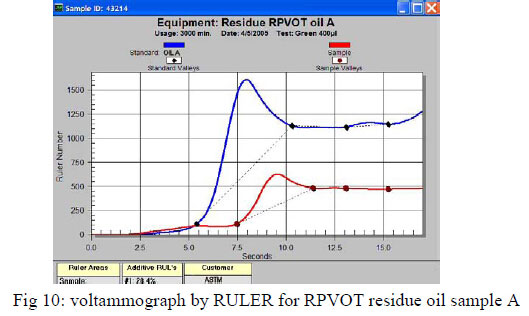
The remaining antioxidant activity in the RPVOT residue oil samples is confirmed for the 3 different oil type (C, B, A - see below respective table 6, 7 & 8) where for the majority of the oxidized oil samples, an important part of the antioxidants is still detected by voltammetry. This data is confirmed by a significantly lower increase of Acid Number and Oxidation, in comparison with the oil samples which have total Antioxidant depletion. The authors leave into discussion why the residue oil sample A have remaining antioxidant with low AN, but with high VPI, whereas oil C has high AN regardless of AO and oil B has low AN regardless of AO. An explanation for this correlation could be found in the type of base oil which has a difference in the ability to dissolve oxidation products, more specifically when moving from Group I to Group III oil, which may affect VPI independent of remaining AO.
5. Conclusions
From the above results, the authors conclude that RPVOT testing of turbine lubricants with single antioxidant systems have much better reproducibility and repeatability than comparable RPVOT testing of oils with complex, synergistic mixtures of antioxidants for both new and used turbine oils obtained from gas and steam turbines.
The results presented in this paper indicate that the effects of antioxidant chemistry on the antioxidant concentration and level of oxidation of the RPVOT test residues help explain the poor reproducibility of the RPVOT tests for turbine oils containing different types of antioxidant systems. Consequently, RULER analyses to characterize the antioxidant systems of the turbine oils before and after RPVOT testing would be very valuable in interpreting the RPVOT results and in improving the reproducibility of the RPVOT technique for making oxidative life assessments of modern generation turbine lubricants.
Based also on the protective function of primary antioxidants in new generation (gas and steam) turbine lubricants, it is of added value to start trending and differentiating the depletion of the individual antioxidants, before excessive base-oil degradation starts to occur with severe known consequences like varnishing and deposit formation. Since identifying a fluid's potential to produce varnish is challenging and routine oil analysis does not provide a sufficient warning, the VPI, when used in conjunction with the RULER antioxidant evaluations, can be useful in predicting the formation of varnishing and deposits. Finally, the authors are convinced that the combination of RULER antioxidant analysis, in conjunction with VPI and RPVOT, will be an important contributor to successful maintenance strategies based on root cause analysis leading to the correct alarm levels and maintenance actions for turbine oils regardless of formulation.

