EXPLANATION
Vapor pressure is an important physical property of liquid spark-ignition engine fuels. It provides an indication of how a fuel will perform under different operating conditions, such as whether it will cause vapor lock at high ambient temperature or at high altitude, or will provide easy starting at low ambient temperature. Petroleum product specifications generally include vapor pressure limits to ensure products of suitable volatility performance. Vapor pressure of fuels is regulated by various government agencies.
This test method is a modification of Reid vapor pressure method Test Method D323 (included here). This test method is applicable to gasolines and gasoline-oxygenate blends with a vapor pressure range of 35 to 100 kPa.
TEST SUMMARY
This test method provides two procedures for determining vapor pressure. The liquid chamber of the vapor pressure apparatus is filled with chilled sample and connected to the vapor chamber at 100° F. The apparatus is immersed in a bath of 100° F until a constant pressure is observed. The pressure reading, suitably corrected, is reported as the vapor pressure.
In Procedure A, the same apparatus and essentially the same procedure as in Test Method D323 is utilized with the exception that the interior surfaces of the liquid and vapor chambers are maintained completely free of water.
Procedure B utilizes a semiautomatic apparatus with the liquid and vapor chambers identical in volume to those in Procedure A. The apparatus is suspended in a horizontal bath and rotated while attaining equilibrium. Either a Bourdon gage or pressure transducer can be used with this procedure. The interior surfaces of the liquid and vapor chambers are maintained free of water.
TEST PRECISION
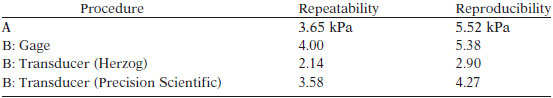
Bias of this test method has not been determined.