Karl Fischer reagent reacts with water as follows:
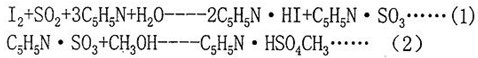
The reagent is a mixture of iodine, pyridine with sulfur dioxide and methanol etc. The iodine is formed on the anode by electrolysis. According to Faraday's law, the same amount of electric charge is proportional, the formula is as follows:
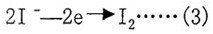
It is easy to see from (1) that in the reaction, the quantity of iodine gram molecules is equal to the quantity of water molecules. After sample is injected into reagent, the moisture in sample starts reaction at once. The consumption of iodine quantity in reaction can be known by the instrument, and the consumption of electric quantity can be known by the consumption of iodine quantity. After calucation of quantity of electricity to electrolyze the same amount of iodine by the instrument, the water content in sample is displayed directly on screen. The instrument adopts electrolytic current automatic cotrol and maximum current reaches 200mA. During process of electrolysis, the moisture content reduces gradually and the titration speed reduces accordingly at a certain proportion until the corresponding titration end point control circuit opens. This system ensures high accuracy and high speed in analysis process. In addition, during measurement process, there will be some interference factors which are hard to avoid, for example, the moisture in air makes titration cell absorb moisture and generate idel current. However, the instrument has the function of memorizing idel current, therefore, the numbers displayed on screen are actual moisture content in sample.

